Abstract
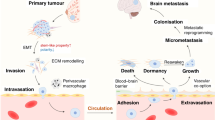
The past decade has witnessed impressive advances in cancer treatment ushered in by targeted and immunotherapies. However, with significantly prolonged survival, upon recurrence, more patients become inflicted by brain metastasis, which is mostly refractory to all currently available therapeutic regimens. Historically, brain metastasis is an understudied area in cancer research, partly due to the dearth of appropriate experimental models that closely simulate the special biological features of metastasis in the unique brain environment and to the sophistication of techniques required to perform in-depth studies of the extremely complex and challenging brain metastasis. Yet, with increasing clinical demand for more effective treatment options, brain metastasis research has rapidly advanced in recent years. The present review spotlights the recent major progresses in basic and translational studies of brain metastasis with focuses on new animal models, novel imaging technologies, omics “big data” resources, and some new and exciting biological insights on brain metastasis.

Similar content being viewed by others
References
Gavrilovic, I. T., & Posner, J. B. (2005). Brain metastases: epidemiology and pathophysiology. Journal of Neuro-Oncology, 75(1), 5–14.
Patchell, R. A. (2003). The management of brain metastases. Cancer Treatment Reviews, 29(6), 533–540.
Barnholtz-Sloan, J. S., Sloan, A. E., Davis, F. G., Vigneau, F. D., Lai, P., & Sawaya, R. E. (2004). Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. Journal of Clinical Oncology, 22(14), 2865–2872.
Schouten, L. J., Rutten, J., Huveneers, H. A., & Twijnstra, A. (2002). Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer, 94(10), 2698–2705.
Perin, T., Canzonieri, V., Memeo, L., & Massarut, S. (2011). Breast metastasis of primary colon cancer with micrometastasis in the axillary sentinel node: a metastasis that metastasized? Diagnostic Pathology, 6, 45.
Janssen, S., Dahlke, M., Trang, N. T., Khoa, M. T., & Rades, D. (2015). Estimation of the six-month survival probability after radiosurgery for brain metastases from kidney cancer. Anticancer Research, 35(7), 4215–4217.
Chua, C., Raaj, J., Pan, S., Farid, M., Lee, J. F., Ho, Z. C., et al. (2016). Brain metastasis in sarcoma: does metastasectomy or aggressive multi-disciplinary treatment improve survival outcomes. Asia-Pacific Journal of Clinical Oncology, 12(1), e16–e22.
Lemke, J., Barth, T. F., Juchems, M., Kapapa, T., Henne-Bruns, D., & Kornmann, M. (2011). Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Research, 31(12), 4599–4603.
Bendell, J. C., Domchek, S. M., Burstein, H. J., Harris, L., Younger, J., Kuter, I., et al. (2003). Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer, 97(12), 2972–2977.
Clayton, A. J., Danson, S., Jolly, S., Ryder, W. D., Burt, P. A., Stewart, A. L., et al. (2004). Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. British Journal of Cancer, 91(4), 639–643.
Kong, W., Jarvis, C., & Mackillop, W. J. (2015). Estimating the need for palliative radiotherapy for brain metastasis: a benchmarking approach. Clinical Oncology (Royal College of Radiologists), 27(2), 83–91.
Tabouret, E., Chinot, O., Metellus, P., Tallet, A., Viens, P., & Goncalves, A. (2012). Recent trends in epidemiology of brain metastases: an overview. Anticancer Research, 32(11), 4655–4662.
Smedby, K. E., Brandt, L., Backlund, M. L., & Blomqvist, P. (2009). Brain metastases admissions in Sweden between 1987 and 2006. British Journal of Cancer, 101(11), 1919–1924.
Steeg, P. S., Camphausen, K. A., & Smith, Q. R. (2011). Brain metastases as preventive and therapeutic targets. Nature Reviews. Cancer, 11(5), 352–363.
Eichler, A. F., Chung, E., Kodack, D. P., Loeffler, J. S., Fukumura, D., & Jain, R. K. (2011). The biology of brain metastases—translation to new therapies. Nature Reviews. Clinical Oncology, 8(6), 344–356.
Zhang, C., & Yu, D. (2011). Microenvironment determinants of brain metastasis. Cell & Bioscience, 1(1), 8.
Nguyen, D. X., Bos, P. D., & Massague, J. (2009). Metastasis: from dissemination to organ-specific colonization. Nature Reviews. Cancer, 9(4), 274–284.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews. Cancer, 3(6), 453–458.
Daphu, I., Sundstrom, T., Horn, S., Huszthy, P. C., Niclou, S. P., Sakariassen, P. O., et al. (2013). In vivo animal models for studying brain metastasis: value and limitations. Clinical & Experimental Metastasis, 30(5), 695–710.
Wu, Y. J., Muldoon, L. L., Gahramanov, S., Kraemer, D. F., Marshall, D. J., & Neuwelt, E. A. (2012). Targeting alphaV-integrins decreased metastasis and increased survival in a nude rat breast cancer brain metastasis model. Journal of Neuro-Oncology, 110(1), 27–36.
Kennecke, H., Yerushalmi, R., Woods, R., Cheang, M. C., Voduc, D., Speers, C. H., et al. (2010). Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology, 28(20), 3271–3277.
Debeb, B. G., Lacerda, L., Anfossi, S., Diagaradjane, P., Chu, K., Bambhroliya, A., et al. (2016). miR-141-mediated regulation of brain metastasis from breast cancer. J Natl Cancer Inst, 108(8).
Ni, J., Ramkissoon, S. H., **e, S., Goel, S., Stover, D. G., Guo, H., et al. (2016). Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nature Medicine, 22(7), 723–726.
Goldberg, S. B., Gettinger, S. N., Mahajan, A., Chiang, A. C., Herbst, R. S., Sznol, M., et al. (2016). Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. The Lancet Oncology, 17(7), 976–983.
Zhang, Y., Zhang, N., Hoffman, R. M., & Zhao, M. (2015). Surgically-induced multi-organ metastasis in an orthotopic syngeneic imageable model of 4T1 murine breast cancer. Anticancer Research, 35(9), 4641–4646.
Erin, N., Kale, S., Tanriover, G., Koksoy, S., Duymus, O., & Korcum, A. F. (2013). Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Research and Treatment, 139(3), 677–689.
Barajas Jr., R. F., & Cha, S. (2012). Imaging diagnosis of brain metastasis. Progress in Neurological Surgery, 25, 55–73.
Waerzeggers, Y., Rahbar, K., Riemann, B., Weckesser, M., Schafers, M., Hesselmann, V., et al. (2010). PET in the diagnosis and management of patients with brain metastasis: current role and future perspectives. Cancer Biomarkers, 7(4), 219–233.
Kienast, Y., von Baumgarten, L., Fuhrmann, M., Klinkert, W. E., Goldbrunner, R., Herms, J., et al. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nature Medicine, 16(1), 116–122.
Murrell, D. H., Zarghami, N., Jensen, M. D., Chambers, A. F., Wong, E., & Foster, P. J. (2016). Evaluating changes to blood-brain barrier integrity in brain metastasis over time and after radiation treatment. Translational Oncology, 9(3), 219–227.
O'Brien, E. R., Kersemans, V., Tredwell, M., Checa, B., Serres, S., Soto, M. S., et al. (2014). Glial activation in the early stages of brain metastasis: TSPO as a diagnostic biomarker. Journal of Nuclear Medicine, 55(2), 275–280.
Poli, G. L., Bianchi, C., Virotta, G., Bettini, A., Moretti, R., Trachsel, E., et al. (2013). Radretumab radioimmunotherapy in patients with brain metastasis: a 124I-L19SIP dosimetric PET study. Cancer Immunology Research, 1(2), 134–143.
Sarmiento, M. (2013). Use of confocal microscopy in the study of microglia in a brain metastasis model. Methods in Molecular Biology, 1041, 337–346.
Saha, D., Dunn, H., Zhou, H., Harada, H., Hiraoka, M., Mason, R. P., et al. (2011). In vivo bioluminescence imaging of tumor hypoxia dynamics of breast cancer brain metastasis in a mouse model. Journal of Visualized Experiments, 56.
Guldner, I. H., Yang, L., Cowdrick, K. R., Wang, Q., Alvarez Barrios, W. V., Zellmer, V. R., et al. (2016). An integrative platform for three-dimensional quantitative analysis of spatially heterogeneous metastasis landscapes. Scientific Reports, 6, 24201.
Chung, K., Wallace, J., Kim, S. Y., Kalyanasundaram, S., Andalman, A. S., Davidson, T. J., et al. (2013). Structural and molecular interrogation of intact biological systems. Nature, 497(7449), 332–337.
Lee, J. Y., Park, K., Lim, S. H., Kim, H. S., Yoo, K. H., Jung, K. S., et al. (2015). Mutational profiling of brain metastasis from breast cancer: matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget, 6(41), 43731–43742.
Lee, H. W., Seol, H. J., Choi, Y. L., Ju, H. J., Joo, K. M., Ko, Y. H., et al. (2012). Genomic copy number alterations associated with the early brain metastasis of non-small cell lung cancer. International Journal of Oncology, 41(6), 2013–2020.
Li, F., Sun, L., & Zhang, S. (2015). Acquirement of DNA copy number variations in non-small cell lung cancer metastasis to the brain. Oncology Reports, 34(4), 1701–1707.
Marzese, D. M., Witz, I. P., Kelly, D. F., & Hoon, D. S. (2015). Epigenomic landscape of melanoma progression to brain metastasis: unexplored therapeutic alternatives. Epigenomics, 7(8), 1303–1311.
Marzese, D. M., Scolyer, R. A., Huynh, J. L., Huang, S. K., Hirose, H., Chong, K. K., et al. (2014). Epigenome-wide DNA methylation landscape of melanoma progression to brain metastasis reveals aberrations on homeobox D cluster associated with prognosis. Human Molecular Genetics, 23(1), 226–238.
Salhia, B., Kiefer, J., Ross, J. T., Metapally, R., Martinez, R. A., Johnson, K. N., et al. (2014). Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PloS One, 9(1), e85448.
Park, E. S., Kim, S. J., Kim, S. W., Yoon, S. L., Leem, S. H., Kim, S. B., et al. (2011). Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proceedings of the National Academy of Sciences of the United States of America, 108(42), 17456–17461.
Camacho, L., Guerrero, P., & Marchetti, D. (2013). MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PloS One, 8(9), e73790.
Yoshida, A., Okamoto, N., Tozawa-Ono, A., Koizumi, H., Kiguchi, K., Ishizuka, B., et al. (2013). Proteomic analysis of differential protein expression by brain metastases of gynecological malignancies. Human Cell, 26(2), 56–66.
Dun, M. D., Chalkley, R. J., Faulkner, S., Keene, S., Avery-Kiejda, K. A., Scott, R. J., et al. (2015). Proteotranscriptomic profiling of 231-BR breast cancer cells: identification of potential biomarkers and therapeutic targets for brain metastasis. Molecular & Cellular Proteomics, 14(9), 2316–2330.
Sjobakk, T. E., Vettukattil, R., Gulati, M., Gulati, S., Lundgren, S., Gribbestad, I. S., et al. (2013). Metabolic profiles of brain metastases. International Journal of Molecular Sciences, 14(1), 2104–2118.
Neagu, M. R., Gill, C. M., Batchelor, T. T., & Brastianos, P. K. (2015). Genomic profiling of brain metastases: current knowledge and new frontiers. Chinese Clinical Oncology, 4(2), 22.
Saunus, J. M., Quinn, M. C., Patch, A. M., Pearson, J. V., Bailey, P. J., Nones, K., et al. (2015). Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. The Journal of Pathology, 237(3), 363–378.
Zhang, L., Zhang, S., Yao, J., Lowery, F. J., Zhang, Q., Huang, W. C., et al. (2015). Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature, 527(7576), 100–104.
Chen, Q., Boire, A., **, X., Valiente, M., Er, E. E., Lopez-Soto, A., et al. (2016). Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature, 533(7604), 493–498.
Paik, P. K., Shen, R., Won, H., Rekhtman, N., Wang, L., Sima, C. S., et al. (2015). Next-generation sequencing of stage IV squamous cell lung cancers reveals an association of PI3K aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discovery, 5(6), 610–621.
Brastianos, P. K., Carter, S. L., Santagata, S., Cahill, D. P., Taylor-Weiner, A., Jones, R. T., et al. (2015). Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discovery, 5(11), 1164–1177.
Li, F., Glinskii, O. V., Zhou, J., Wilson, L. S., Barnes, S., Anthony, D. C., et al. (2011). Identification and analysis of signaling networks potentially involved in breast carcinoma metastasis to the brain. PloS One, 6(7), e21977.
**ng, F., Sharma, S., Liu, Y., Mo, Y. Y., Wu, K., Zhang, Y. Y., et al. (2015). miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-alpha. Oncogene, 34(37), 4890–4900.
Ahmad, A., Sethi, S., Chen, W., Ali-Fehmi, R., Mittal, S., & Sarkar, F. H. (2014). Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. American Journal of Translational Research, 6(4), 384–390.
Okuda, H., **ng, F., Pandey, P. R., Sharma, S., Watabe, M., Pai, S. K., et al. (2013). miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Research, 73(4), 1434–1444.
Hwang, S. J., Seol, H. J., Park, Y. M., Kim, K. H., Gorospe, M., Nam, D. H., et al. (2012). MicroRNA-146a suppresses metastatic activity in brain metastasis. Molecules and Cells, 34(3), 329–334.
Zhang, L., Sullivan, P. S., Goodman, J. C., Gunaratne, P. H., & Marchetti, D. (2011). MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Research, 71(3), 645–654.
Donzelli, S., Mori, F., Bellissimo, T., Sacconi, A., Casini, B., Frixa, T., et al. (2015). Epigenetic silencing of miR-145-5p contributes to brain metastasis. Oncotarget, 6(34), 35183–35201.
Hwang, S. J., Lee, H. W., Kim, H. R., Song, H. J., Lee, D. H., Lee, H., et al. (2015). Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget, 6(24), 20434–20448.
Chen, L. T., Xu, S. D., Xu, H., Zhang, J. F., Ning, J. F., & Wang, S. F. (2012). MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Medical Oncology, 29(3), 1673–1680.
Singh, M., Garg, N., Venugopal, C., Hallett, R., Tokar, T., McFarlane, N., et al. (2015). STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget, 6(29), 27461–27477.
Hanniford, D., Zhong, J., Koetz, L., Gaziel-Sovran, A., Lackaye, D. J., Shang, S., et al. (2015). A miRNA-based signature detected in primary melanoma tissue predicts development of brain metastasis. Clinical Cancer Research, 21(21), 4903–4912.
Nasser, S., Ranade, A. R., Sridhar, S., Haney, L., Korn, R. L., Gotway, M. B., et al. (2011). Biomarkers associated with metastasis of lung cancer to brain predict patient survival. International Journal of Data Mining and Bioinformatics, 5(3), 287–307.
Wu, K., Sharma, S., Venkat, S., Liu, K., Zhou, X., & Watabe, K. (2016). Non-coding RNAs in cancer brain metastasis. Frontiers in Bioscience (Scholar Edition), 8, 187–202.
Shen, L., Chen, L., Wang, Y., Jiang, X., **a, H., & Zhuang, Z. (2015). Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. Journal of Neuro-Oncology, 121(1), 101–108.
de Oliveira Barros, E. G., Palumbo Jr., A., Mello, P. L., de Mattos, R. M., da Silva, J. H., Pontes, B., et al. (2014). The reciprocal interactions between astrocytes and prostate cancer cells represent an early event associated with brain metastasis. Clinical & Experimental Metastasis, 31(4), 461–474.
Klein, A., Schwartz, H., Sagi-Assif, O., Meshel, T., Izraely, S., Ben Menachem, S., et al. (2015). Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. The Journal of Pathology, 236(1), 116–127.
Valiente, M., Obenauf, A. C., **, X., Chen, Q., Zhang, X. H., Lee, D. J., et al. (2014). Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 156(5), 1002–1016.
Jia, W., Martin, T. A., Zhang, G., & Jiang, W. G. (2013). Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Research, 33(6), 2353–2359.
Blecharz, K. G., Colla, R., Rohde, V., & Vajkoczy, P. (2015). Control of the blood-brain barrier function in cancer cell metastasis. Biology of the Cell, 107(10), 342–371.
Wilhelm, I., Molnar, J., Fazakas, C., Hasko, J., & Krizbai, I. A. (2013). Role of the blood-brain barrier in the formation of brain metastases. International Journal of Molecular Sciences, 14(1), 1383–1411.
Weidle, U. H., Niewohner, J., & Tiefenthaler, G. (2015). The blood-brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genomics Proteomics, 12(4), 167–177.
Winkler, F., Osswald, M., Blaes, J., Liao, Y., Solecki, G., Gommel, M., et al. (2016). Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res.
Fortin, D. (2012). The blood-brain barrier: its influence in the treatment of brain tumors metastases. Current Cancer Drug Targets, 12(3), 247–259.
Wrobel, J. K., & Toborek, M. (2016). Blood-brain barrier remodeling during brain metastasis formation. Mol Med.
Yonemori, K., Tsuta, K., Ono, M., Shimizu, C., Hirakawa, A., Hasegawa, T., et al. (2010). Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer, 116(2), 302–308.
Connell, J. J., Chatain, G., Cornelissen, B., Vallis, K. A., Hamilton, A., Seymour, L., et al. (2013). Selective permeabilization of the blood-brain barrier at sites of metastasis. Journal of the National Cancer Institute, 105(21), 1634–1643.
Adkins, C. E., Mohammad, A. S., Terrell-Hall, T. B., Dolan, E. L., Shah, N., Sechrest, E., et al. (2016). Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clinical & Experimental Metastasis, 33(4), 373–383.
Lyle, L. T., Lockman, P. R., Adkins, C. E., Mohammad, A. S., Sechrest, E., Hua, E., et al. (2016). Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res.
Zhang, S., Huang, W. C., Zhang, L., Zhang, C., Lowery, F. J., Ding, Z., et al. (2013). SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Research, 73(18), 5764–5774.
Do, J., Foster, D., Renier, C., Vogel, H., Rosenblum, S., Doyle, T. C., et al. (2014). Ex vivo Evans blue assessment of the blood brain barrier in three breast cancer brain metastasis models. Breast Cancer Research and Treatment, 144(1), 93–101.
Li, J., Cai, P., Shalviri, A., Henderson, J. T., He, C., Foltz, W. D., et al. (2014). A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano, 8(10), 9925–9940.
Wan, X., Zheng, X., Pang, X., Pang, Z., Zhao, J., Zhang, Z., et al. (2016). Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget.
Kobus, T., Zervantonakis, I. K., Zhang, Y., & McDannold, N. J. (2016). Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. Journal of Controlled Release, 238, 281–288.
Acknowledgments
This work was supported partially by R01CA112567-06 (DY), R01CA184836 (DY), METAvivor Research Grant (DY), and China Medical University Research Fund (DY). D. Yu is the Hubert L. & Olive Stringer Distinguished Chair in Basic Science at MDACC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Note:
We apologize for not being able to cite all the relevant original research and review articles due to space limitation.
Rights and permissions
About this article
Cite this article
Zhang, C., Yu, D. Advances in decoding breast cancer brain metastasis. Cancer Metastasis Rev 35, 677–684 (2016). https://doi.org/10.1007/s10555-016-9638-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9638-9