Abstract
Purpose
Various studies have demonstrated the causal relationship between gut microbiota and efficacy of chemotherapy; however, the impact of gut microbiota on breast cancer has not been fully elucidated. This study aimed to evaluate the associations between the gut microbiota before neoadjuvant chemotherapy and its consequent efficacy in breast cancer.
Methods
This prospective observational study included patients who received neoadjuvant chemotherapy for primary early breast cancer at eight institutions between October 1, 2019, and March 31, 2022. We performed 16S rRNA analysis of fecal samples and α and β diversity analyses of the gut microbiota. The primary endpoint was the association between the gut microbiota and pathological complete response (pCR) to neoadjuvant chemotherapy.
Results
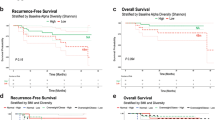
Among the 183 patients, the pCR rate after neoadjuvant chemotherapy was 36.1% in all patients and 12.9% (9/70), 69.5% (41/59), and 29.6% (16/54) in those with the luminal, human epidermal growth factor receptor 2, and triple-negative types, respectively. The α diversity of the gut microbiota did not significantly differ between patients with pCR and those without pCR. Among the gut microbiota, two species (Victivallales, P = 0.001 and Anaerolineales, P = 0.001) were associated with pCR, and one (Gemellales, P = 0.002) was associated with non-pCR.
Conclusion
Three species in the gut microbiota had potential associations with neoadjuvant chemotherapy efficacy, but the diversity of the gut microbiota was not associated with response to chemotherapy. Further research is needed to validate our findings.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the Setouchi Breast Project Comprehensive Support Organization but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Setouchi Breast Project Comprehensive Support Organization.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org (2019) Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(8):1194–1220. https://doi.org/10.1093/annonc/mdz173
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Luen SJ, Savas P, Fox SB, Salgado R, Loi S (2017) Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 49(2):141–155. https://doi.org/10.1016/j.pathol.2016.10.010
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29(15):1949–1955. https://doi.org/10.1200/JCO.2010.30.5037
Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY (2013) Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 109(10):2705–2713. https://doi.org/10.1038/bjc.2013.634
de Vos WM, de Vos EA (2012) Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev 70(Suppl 1):S45–S56. https://doi.org/10.1111/j.1753-4887.2012.00505.x
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359(6371):97–103. https://doi.org/10.1126/science.aan4236
Di Modica M, Gargari G, Regondi V, Bonizzi A, Arioli S, Belmonte B, De Cecco L, Fasano E, Bianchi F, Bertolotti A, Tripodo C, Villani L, Corsi F, Guglielmetti S, Balsari A, Triulzi T, Tagliabue E (2021) Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res 81(8):2195–2206. https://doi.org/10.1158/0008-5472.CAN-20-1659
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342(6161):967–970. https://doi.org/10.1126/science.1240527
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342(6161):971–976. https://doi.org/10.1126/science.1240537
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350(6264):1084–1089. https://doi.org/10.1126/science.aac4255
Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, Vaysse T, Marthey L, Eggermont A, Asvatourian V, Lanoy E, Mateus C, Robert C, Carbonnel F (2017) Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 28(6):1368–1379. https://doi.org/10.1093/annonc/mdx108
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF (2018) The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359(6371):104–108. https://doi.org/10.1126/science.aao3290
Heshiki Y, Vazquez-Uribe R, Li J, Ni Y, Quainoo S, Imamovic L, Li J, Sørensen M, Chow BK, Weiss GJ, Xu A, Sommer MO, Panagiotou G (2020) Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome 8(1):28. https://doi.org/10.1186/s40168-020-00811-2
Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T (2020) The Japanese breast cancer society clinical practice guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer 27(3):322–331. https://doi.org/10.1007/s12282-020-01085-0
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Pathologists (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TILs Working Group 2014 (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017) Possible role of the gut microbiota–brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry 7(12):1294. https://doi.org/10.1038/s41398-017-0031-4
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CP, Flament C, Lepage P, Roberti MP, Routy B, Jacquelot N, Apetoh L, Becharef S, Rusakiewicz S, Langella P, Sokol H, Kroemer G, Enot D, Roux A, Eggermont A, Tartour E, Johannes L, Woerther PL, Chachaty E, Soria JC, Golden E, Formenti S, Plebanski M, Madondo M, Rosenstiel P, Raoult D, Cattoir V, Boneca IG, Chamaillard M, Zitvogel L (2016) Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45(4):931–943. https://doi.org/10.1016/j.immuni.2016.09.009
Westman EL, Canova MJ, Radhi IJ, Koteva K, Kireeva I, Waglechner N, Wright GD (2012) Bacterial inactivation of the anticancer drug doxorubicin. Chem Biol 19(10):1255–1264. https://doi.org/10.1016/j.chembiol.2012.08.011
Li Y, Dong B, Wu W, Wang J, ** H, Chen K, Huang K, Huang S, Yao Y (2022) Metagenomic analyses reveal distinct gut microbiota signature for predicting the neoadjuvant chemotherapy responsiveness in breast cancer patients. Front Oncol 12:865121. https://doi.org/10.3389/fonc.2022.865121
Aarnoutse R, Ziemons J, Hillege LE, de Vos-Geelen J, de Boer M, Bisschop SM, Vriens BE, Vincent J, van de Wouw AJ, Le GN, Venema K, Rensen SS, Penders J, Smidt ML (2022) Changes in intestinal microbiota in postmenopausal oestrogen receptor-positive breast cancer patients treated with (neo)adjuvant chemotherapy. NPJ Breast Cancer 8(1):89. https://doi.org/10.1038/s41523-022-00455-5
He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, Mujagond P, Chen XJ, Rong ZH, Chen P, Lyu LY, Wang X, Wu CB, Yu N, Xu YJ, Yin J, Raes J, Knight R, Ma WJ, Zhou HW (2018) Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med 24(10):1532–1535. https://doi.org/10.1038/s41591-018-0164-x
McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM, Fernandes MR, Costa RG, Yuan W, Salcedo R, Bahadiroglu E, Roy S, DeBlasio RN, Morrison RM, Chauvin JM, Ding Q, Zidi B, Lowin A, Chakka S, Gao W, Pagliano O, Ernst SJ, Rose A, Newman NK, Morgun A, Zarour HM, Trinchieri G, Dzutsev AK (2022) Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med 28(3):545–556. https://doi.org/10.1038/s41591-022-01698-2
Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, Zidi B, Zhang S, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RG, Balaji AK, Morgun A, Vujkovic-Cvi** I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM (2021) Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371(6529):595–602. https://doi.org/10.1126/science.abf3363
Bycura D, Santos AC, Shiffer A, Kyman S, Winfree K, Sutliffe J, Pearson T, Sonderegger D, Cope E, Caporaso JG (2021) Impact of different exercise modalities on the human gut microbiome. Sports 9(2):14. https://doi.org/10.3390/sports9020014
Clauss M, Gérard P, Mosca A, Leclerc M (2021) Interplay between exercise and gut microbiome in the context of human health and performance. Front Nutr 8:637010. https://doi.org/10.3389/fnut.2021.637010
Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W (2010) Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 5(5):e10667. https://doi.org/10.1371/journal.pone.0010667
Hou MF, Ou-Yang F, Li CL, Chen FM, Chuang CH, Kan JY, Wu CC, Shih SL, Shiau JP, Kao LC, Kao CN, Lee YC, Moi SH, Yeh YT, Cheng CJ, Chiang CP (2021) Comprehensive profiles and diagnostic value of menopausal-specific gut microbiota in premenopausal breast cancer. Exp Mol Med 53(10):1636–1646. https://doi.org/10.1038/s12276-021-00686-9
Fan HN, Zhu P, Lu YM, Guo JH, Zhang J, Qu GQ, Zhu JS (2020) Mild changes in the mucosal microbiome during terminal ileum inflammation. Microb Pathog 142:104104. https://doi.org/10.1016/j.micpath.2020.104104
Sun P, Li Z, Zhang B (2023) Characterization of disease-associated microbiota in hepatocellular carcinoma. J Cancer Res Ther 19(4):881–891. https://doi.org/10.4103/jcrt.jcrt_139_22
Cheng X, Wang J, Gong L, Dong Y, Shou J, Pan H, Yu Z, Fang Y (2022) Composition of the gut microbiota associated with the response to immunotherapy in advanced cancer patients: a Chinese real-world pilot study. J Clin Med 11(18):5479. https://doi.org/10.3390/jcm11185479
Acknowledgements
We would like to thank Ms. Miki Fujiwara, Ms. Mayumi Doumae, and Ms. Eriko Ishioka at the Setouchi Breast Project Comprehensive Support Organization. The results were presented in Japan at the 2023 Breast Cancer Society Regional Meeting and in Italy at the 14th European Breast Cancer Conference. We would like to thank Enago (www.enago.com) for editing the draft of this manuscript.
Funding
This study was conducted as a research support project for the Setouchi Breast Project Comprehensive Support Organization.
Author information
Authors and Affiliations
Contributions
NT, YK, MI, HD, and TI contributed to the study conception and design. SN, NT, YK, YM, TK, MY, DT, SK, HH, YO, HD, and TI prepared the material preparation and collected the data. AH and KT performed pathological studies of the specimens in this study. SN and TI performed the formal analysis. SN, NT, MI, HD, TS, TI, and ST contributed to the interpretation and discussion of the results. SN wrote the first draft of the manuscript, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SN: Lecture fee: Daiichi-Sankyo, Eisai and Chugai; AH received honoraria from Daiichi-Sankyo, Pfizer, Chugai and is a member of the Advisory Board of Daiichi-Sankyo, Exact Science; Research grant: Visiopharm, MI: Research grant: Lilly, Honoraria: Daiichi-Sankyo, Lilly, Pfizer, Ezai, Kyowa Kirin, Astra Zeneca, Taiho, Celltrion Healthcare Japan, MSD, Tsumura, Nihon Mediphysics, Exact Science, Chugai, Nihon Kayaku; Advisory Board: Daiichi-Sankyo; Manuscript fee: Pfizer, Chugai, Medic Media; TI received a research grant from Pfizer. The other authors declare no conflicts of interest associated with this manuscript.
Consent to participate and publish
Informed consent was obtained from all individual participants included in the study. All authors consent to the publication of this manuscript. The patient informed consent also included a section on consenting to the publication of the patients’ anonymized data.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study received an approval from the Ethics Committee of Okayama University Hospital (1909-032, October, 4 2019) and the respective institution, and each participant provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2024_7395_MOESM1_ESM.tif
Supplemental Figure 1. α-diversity of the gut microbiome by breast cancer subtypes with (a) Shannon and (b) Chao 1. Abbreviations: HER2, human epidermal growth factor receptor type2; pCR, pathological complete response; TN, triple negative. (TIF 2569 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakamoto, S., Kajiwara, Y., Taniguchi, K. et al. Baseline gut microbiota as a predictive marker for the efficacy of neoadjuvant chemotherapy in patients with early breast cancer: a multicenter prospective cohort study in the Setouchi Breast Project-14. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07395-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07395-7