Abstract
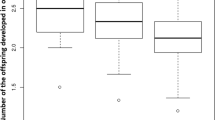
Understanding how native hyperparasitoids are attuned to novel hosts has practical implications for the classical biological control of invasive pest insects. This research focuses on both host size and female parasitoid body size to examine their joint effects on host evaluation and offspring performance. The parasitoid is Cheiloneurus nankingensis, which is native to Asia and an obligate gregarious hyperparasitoid attacking Aenasius arizonensis, which parasitizes the mealybug Phenacoccus solenopsis invasive to Asia. There was a quadratic ‘dome-shaped’ relationship between mealybug body size and both antenna-tap** and ovipositor-drilling frequencies and durations. Parasitoid body size did not affect these behaviors, except having a slight negative effect on ovipositor-drilling duration. Offspring egg-to-adult development time was similar for both large and small females when attacking large mealybugs, but it was longer for large females compared to small ones when attacking small mealybugs. Brood size at eclosion increased with both mealybug size and female size. Brood sex ratio (percent males) decreased with mealybug size while remaining unaffected by female size. The body size of adult offspring and their longevity increased with mealybug size while remaining unaffected by female size. These findings imply that mealybug size is much more relevant than female size to host examination behaviors and both aspects of size exert separate effects on brood allocation but an interaction to affect offspring developmental time.




Similar content being viewed by others
References
Aartsma Y, Cusumano A, Fernández de Bobadilla MF, Rusman Q, Vosteen I, Poelman EH (2019) Understanding insect foraging in complex habitats by comparing trophic levels: insights from specialist host-parasitoid-hyperparasitoid systems. Curr Opin Insect Sci 32:54–60
Adamo SA (2022) Dividing up the bill: interactions between how parasitoids. Manipulate host behaviour and who pays the cost. Func Ecol 37:801–808
Aga TM, Tambe VJ, Nagrare VS, Naikwadi B (2016) Parasitoid, Aenasius arizonensis (Girault) (Hymenoptera: Encyrtidae): its biology, morphometrics, host stage preference and use in biological control. J Biol Control 30:91–98
Ashfaq M, Shah GS, Noor AR, Ansari SP, Mansoor S (2010) Report of a parasitic wasp (Hymenoptera: Encyrtidae) parasitizing cotton mealybug (hemiptera: Pseudococcidae) in Pakistan and use of PCR for estimating parasitism levels. Biocontrol Sci Techn 20:625–630
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330
Charnov EL, Skinner SW (1984) Evolution of host selection and clutch size in parasitoid wasps. Fla Entomol 67:5–21
Charnov EL, Skinner SW (1988) Clutch size in parasitoids: the egg production rate as a constraint. Evol Ecol 2:167–174
Chen HY, Cao RX, Xu ZF (2010) First record of Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae), a parasitoid of the mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) from China. J Environ Entomol 32:280–282
Chen HY, Li HL, Pang H, Zhu CD, Zhang YZ (2021) Investigating the parasitoid community associated with the invasive mealybug Phenacoccus solenopsis in southern China. Insects 12:290
Cohen JE, Jonsson T, Müller CB, Godfray HCJ, Savage VM (2005) Body sizes of hosts and parasitoids in individual feeding relationships. Proc Natl Acad Sci U S A 102:684–689
Crawley MJ (2007) The R book. Wiley, Chichester
Dorn S, Beckage NE (2007) Superparasitism in gregarious hymenopteran parasitoids: ecological, behavioural and physiological perspectives. Physiol Entomol 32:199–211
Fallahzadeh M, Japoshvili G, Abdimaleki R, Saghaei N (2014) New records of Tetracneminae (Hymenoptera, Chalcidoidea, Encyrtidae) from Iran. Turkish J Zool 38:515–518
Gao SK, Wei K, Tang ZL, Wang XY, Yang ZQ (2016) Effect of parasitoid density on the timing of parasitism and development duration of progeny in Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol Control 97:57–62
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton Univeristy Press, New Jersey
Hardy ICW, Griffiths NT, Godfray HCJ (1992) Clutch size in a parasitoid wasp: a manipulation experiment. J Anim Ecol 61:121–129
Hardy ICW, Smith DR (2023) Statistical approaches. In: Hardy ICW, Wainberg E (eds) Jervis’s insects as natural enemies: practical perspectives. Springer, London, pp 705–741
Harvey JA, Harvey IF, Thompson DJ (2001) Lifetime reproductive success in the solitary endoparasitoid, Venturia canescens. J Insect Behav 14:573–593
Harvey JA (2005) Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol Exp Appl 117:1–13
Harvey JA, Strand MR (2002) The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology 83:2439–2451
Harvey JA, Poelman EH, Tanaka T (2013) Intrinsic and inter- and intraspecific competition in parasitoid wasps. Annu Rev Entomol 58:333–351
Hayat M (2009) Description of a new species of Aenasius Walker (Hymenoptera: Encyrtidae), parasitoid of the mealybug, Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) in India. Biosystematica 3:21–26
He LF, Feng DD, Li P, Xu ZF (2012) Host-instar selection of Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae) for mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). J Environ Entomol 34:329–333
Henri DC, Van Veen FJF (2011) Body size, life history and the structure of host-parasitoid networks. Adv Ecol Res 45:135–180
Herren HR, Neuenschwander P (1991) Biological control of cassava pests in Africa. Annu Rev Entomol 36:257–283
Hodgson C, Abbas G, Arif MJ, Saeed S, Karar H (2008) Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa 1913:1–35
Jervis MA, Ellers J, Harvey JA (2008) Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu Rev Entomol 53:361–385
Juliano SA (1985) The effects of body size on mating and reproduction in Brachnus lateralis (Coleoptera: Carabidae). Ecol Entomol 10:271–280
Kapranas A, Tena A (2015) Encyrtid parasitoids of soft scale insects: biology, behavior, and their use in biological control. Annu Rev Entomol 60:195–211
Kazmer DJ, Luck RF (1995) Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma pretiosum. Ecology 76:412–425
Khuhro SN, Kalroo AM, Mahmood R (2011) Present status of mealy bug Phenacoccus solenopsis (Tinsley) on cotton and other plants in Sindh (Pakistan). In: Kranthi KD, Venugopalan MV, Balasubramanya RH, Kranthi S, Singh S, Blaise (eds) Book of papers world cotton research conference-5. Excel India Publishers, New Delhi, pp 268–271
Klomp H, Teerink BJ (1962) Host selection and number of eggs per oviposition in the egg-parasite Trichogramma embryophagum Htg. Nature 195:1020–1021
Li JF, Deng J, Chen HY, Yang L, Zhou ZS, Jiang JJ, Huang LF, Gui FR, Chen HS (2020a) Investigation on the occurrence of parasitic wasps of Phenacoccus solenopsis Tinsley in Guangxi. J Southern Agric 51:853–861
Li ZM, Yao TT, Xu ZH, Meng L, Li BP (2020b) A new species of cheiloneurus westwood (Hymenoptera, encyrtidae) as a hyperparasitoid of the invasive cotton mealybug, phenacoccus solenopsis Tinsley, in China. Zookeys 2020:23–29
Libersat F, Delago A, Gal R (2009) Manipulation of host behavior by parasitic insects and insect parasites. Annu Rev Entomol 54:189–207
Mackauer M, Sequeira R (1993) Patterns of development in insect parasites. In: Beckage NE, Thompson SN, Federici BA (eds) Parasites and pathogens of insects. Academic Press, Orlando, pp 1–23
Mahmood R (2008) Breakthrough in biological control of mealybug in Pakistan. Biocontrol News Inform 29:38–39
Matokot L, Reyd G, Malonga P, Le Rü B (1992) Population dynamics of Rastrococcus invadens (Homoptera: Pseudococcidae) in Congo; influence of accidental introduction of the Asiatic parasitoid Gyranusoidea tebygi (Hymenoptera: Encyrtidae). Entomophaga 37:123–140
Moore D (2004) Biological control of Rastrococcus invadens. Biocontrol News Inform 25:17–27
Murray D, Charleston K (2010) Exotic mealybug species—a major new pest in cotton. https://thebeatsheet.com.au/exotic-mealybug-species-a-major-new-pest-in-cotton. Accessed 12 February 2010
Neuenschwander P (2001) Biological control of the cassava mealybug in Africa: a review. Biol Control 21:214–229
Noureen N, Hussain M, Fatima S, Ghazanfar M (2016) Cotton mealybug management: a review. J Entomol Zool Stud 4:657–663
Petersen G, Hardy ICW (1996) The importance of being larger: parasitoid intruder-owner contests and their implications for clutch size. Anim Behav 51:1363–1373
Qin WQ, Lyu YJ, Yao TT, Meng L, Li BP (2023) Host stage affects oviposition-related behaviours, development progression and reproductive output in a native hyperparasitoid of the solenopsis mealybug invading Asian regions. Biocontrol Sci Techn 33:789–804
Quicke DLJ (1997) Parasitic wasps. Chapman & Hall, London
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.r-project.org/
Ram P, Saini RK, Vijaya (2009) Preliminary studies on field parasitization and biology of solenopsis mealybug parasitoid, Aenasius bambawalei Hayat (Encyrtidae: Hymenoptera). J Cott Res Dev 23:313–315
Rasekh A, Ameri M, Atashdar H (2022) The contribution of maternal and paternal body size to offspring early adulthood life histories in a parasitoid wasp, Lysiphlebus fabarum. Evol Ecol 36:409–420
Samková A, Hadrava J, Skuhrovec J, Janšta P (2019) Reproductive strategy as a major factor determining female body size and fertility of a gregarious parasitoid. J Appl Entomol 143:441–450
Schmidt JM, Smith JJB (1985) Host volume measurement by the parasitoid wasp Trichogramma minutum: the roles of curvature and surface area. Entomol Exp Appl 39:213–221
Segoli M, Rosenheim JA (2015) The effect of body size on oviposition success of a minute parasitoid in nature. Ecol Entomol 40:483–485
Spodek M, Ben-Dov Y, Mondaca L, Protasov A, Erel E, Mendel Z (2018) The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in Israel: pest status, host plants and natural enemies. Phytoparasitica 46:45–55
Strand MR (2000) Developmental traits and life-history evolution in parasitoids. In: Hochberg ME, Ives AR (eds) Parasitoid population biology. Princeton University Press, Princeton, pp 139–162
Strand MR, Casas J (2008) Parasitoid and host nutrtional physiology in behavioral ecology. In: Wajnberg E, Bernstein C, van Alphen J (eds) Behavioral ecology of insect parasitoids: from theoretical approaches to field application. Blackwell, Oxford, pp 113–128
Takagi M (1985) The reproductive strategy of the gregarious parasitoid, Pteromalus puparum (Hymenoptera: Pteromalidae)−1. Optimal number of eggs in a single host. Oecologia 68:1–6
Takagi M (1986) The reproductive strategy of the gregarious parasitoid, Pteromalus puparum (Hymenoptera: Pteromalidae)−2. Host size discrimination and regulation of the number and sex ratio of progeny in a single host. Oecologia 70:321–325
Therneau T (2023) A package for survival analysis in R. R package version 3.5-3. https://CRAN.R-project.org/package=survival
Tong HJ, Ao Y, Li ZH, Wang Y, Jiang MX (2019) Invasion biology of the cotton mealybug, Phenacoccus solenopsis Tinsley: current knowledge and future directions. J Integr Agric 18:758–770
Tougeron K, Tena A (2019) Hyperparasitoids as new targets in biological control in a global change context. Biol Control 130:164–171
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452
Vet LEM, Datema A, Janssen A, Snellen H (1994) Clutch size in a larval-pupal endoparasitoid: consequences for fitness. J Anim Ecol 63:807–815
Vinson SB, Iwantsch GF (1980) Host suitability for insect parasitoids. Annu Rev Entomol 25:397–419
Visser ME (1994) The importance of being large: the relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J Anim Ecol 63:963–978
Vos M, Hemerik L (2003) Linking foraging behavior to lifetime reproductive success for an insect parasitoid: adaptation to host distributions. Behav Ecol 14:236–245
Waage JK, Godfray HCJ (1985) Reproductive strategies and population ecology of insect parasitoids. In: Sibly RM, Smith RH (eds) Behavioural ecology. Blackwell, Oxford, pp 449–470
Wang YS, Dai TM, Tian H, Wan FH, Zhang GF (2020) Range expansion of the invasive cotton mealybug, Phenacoccus solenopsis Tinsley: an increasing threat to agricultural and horticultural crops in China. J Integr Agric 19:881–885
Waqas MS, Shi Z, Yi TC, **ao R, Shoaib AA, Elabasy AS, ** DC (2021) Biology, ecology, and management of cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Pest Manag Sci 77:5321–5333
Webster B, Cardé RT (2017) Use of habitat odour by host-seeking insects. Biol Rev 92:1241–1249
Weinersmith KL (2019) What’s gotten into you?: a review of recent research on parasitoid manipulation of host behavior. Curr Opin Insect Sci 33:37–42
Weseloh RM (1969) Biology of Cheiloneurus noxius, with emphasis on host relationships and oviposition behavior. Ann Entomol Soc Am 62:299–305
West S (2009) Sex allocation. Princeton University Press, Princeton
Williams DJ, de Willink MC (1992) Mealybugs of central and south America. CAB International, Wallingford
Yao TT, Qin WQ, Meng L, Li BP (2022) Oviposition and developmental performances of the gregarious hyperparasitoid Cheiloneurus nankingensis in relation to host age. Biol Control 172:104967
Zeya SB, Mohan M, Anwar PT, Narayanan A (2022) First record and redescription of hyperparasitoid of cotton mealybug, Cheiloneurus nankingensis Li & Xu (Hymenoptera: Encyrtidae) from India with some other records. J Insect Biodivers 34:5–11
Acknowledgements
We thank Dr. Ian C. W. Hardy for his valuable suggestions in data analysis and help in straightening up the manuscript. We also thank Qi Guo and Yujia Lyu for their help in rearing insects. This work was supported by the National Key Research and Development Program of China (2017YFE0104900) awarded to L. M.
Funding
Funding was provided by Ministry of Science and Technology of the People’s Republic of China (Grant No. 2017YFE0104900).
Author information
Authors and Affiliations
Contributions
BL and LM designed the study. WQ and TY collected and analyzed the data. WQ and BL wrote the manuscript. All authors reviewed and approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
There is no conflict of interest between all the authors.
Ethical approval
No ethical approval is required.
Additional information
Handling Editor: Stefano Colazza.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, W., Yao, T., Meng, L. et al. Effects of mealybug and female body sizes on host examination and offspring developmental performances in the gregarious hyperparasitoid Cheiloneurus nankingensis. BioControl (2024). https://doi.org/10.1007/s10526-024-10274-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10526-024-10274-1