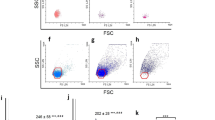
Murine peritoneal macrophages isolated from the lavage fluid after administration of thioglycolate and concanavalin A are presented by two populations of cells of different diameters. Polarization of macrophages into a proinflammatory (M1) phenotype is accompanied by an increase in number of small cells. Macrophages obtained after administration of thioglycolate demonstrate higher tendency to anti-inflammatory (M2) phenotype, while macrophages isolated after administration of concanavalin A are committed in the proinflammatory direction. Lactate level is increased in M1 macrophages in comparison with M2 cells, which indicates predominance of glycolytic metabolism. Macrophages obtained after administration of concanavalin A have reduced mitochondrial potential, which reflects a tendency to apoptosis. Autophagy activation and inhibition neutralize the differences in pro- and anti-inflammatory properties of polarized macrophages obtained after thioglycolate administration, but have less pronounced effect on macrophages obtained after administration concanavalin A. Autophagy inhibitor increases mitochondrial potential in non-polarized macrophages obtained after administration of concanavalin A. These results demonstrate divergent properties of macrophages obtained after administration of glycolate and concanavalin A due to the difference in the mechanisms of experimental peritonitis.
Similar content being viewed by others
References
Bonilla DL, Bhattacharya A, Sha Y, Xu Y, **ang Q, Kan A, Jagannath C, Komatsu M, Eissa NT. Autophagy regulates phagocytosis by modulating the expression of scavenger receptors. Immunity. 2013;39(3):537-547. doi: https://doi.org/10.1016/j.immuni.2013.08.026
Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am. J. Pathol. 2001;158(2):715-721. doi: https://doi.org/10.1016/S0002-9440(10)64014-X
Cassado Ados A, D’Império Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: Heterogeneity, development, and function. Front. Immunol. 2015;6:225. doi: https://doi.org/10.3389/fimmu.2015.00225
Cook AD, Braine EL, Hamilton JA. The Phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J. Immunol. 2003;171(9):4816-4823. doi: https://doi.org/10.4049/jimmunol.171.9.4816
Gordon S, Plüddemann A. Tissue macrophages: Heterogeneity and functions. BMC Biol. 2017;15(1):53. doi: https://doi.org/10.1186/s12915-017-0392-4
Lai YC, Chuang YC, Chang CP, Yeh TM. Macrophage migration inhibitory factor has a permissive role in concanavalin A-induced cell death of human hepatoma cells through autophagy. Cell Death Dis. 2015;6(12):e2008. doi: https://doi.org/10.1038/cddis.2015.349
Liu T, Wang L, Liang P, Wang X, Liu Y, Cai J, She Y, Wang D, Wang Z, Guo Z, Bates S, **a X, Huang J, Cui J. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell. Mol. Immunol. 2020. Oct. 23. doi: https://doi.org/10.1038/s41423-020-00567-7
Liu Z. Thioglycollate Induced Peritonitis. Bio-Protocol. 2011;1(12):18-20. doi:https://doi.org/10.21769/BioProtoc.84
Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. doi: https://doi.org/10.1038/nature06639
Pavlou S, Wang L, Xu H, Chen M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J. Inflamm. (Lond). 2017;14:4. doi: https://doi.org/10.1186/s12950-017-0151-x
Pratt J, Annabi B. Induction of autophagy biomarker BNIP3 requires a JAK2/STAT3 and MT1-MMP signaling interplay in Concanavalin-A-activated U87 glioblastoma cells. Cell Signal. 2014;26(5):917-924. doi: https://doi.org/10.1016/j.cellsig.2014.01.012
Ruiz-Alcaraz AJ, Carmona-Martínez V, Tristán-Manzano M, Machado-Linde F, Sánchez-Ferrer ML, García-Peñarrubia P, Martínez-Esparza M. Characterization of human peritoneal monocyte/macrophage subsets in homeostasis: Phenotype, GATA6, phagocytic/oxidative activities and cytokines expression. Sci. Rep. 2018;8(1):12794. doi: https://doi.org/10.1038/s41598-018-30787-x
Shan M, Qin J, ** F, Han X, Guan H, Li X, Zhang J, Zhang H, Wang Y. Autophagy suppresses isoprenaline-induced M2 macrophage polarization via the ROS/ERK and mTOR signaling pathway. Free Radic. Biol. Med. 2017;110:432-443. doi: https://doi.org/10.1016/j.freeradbiomed.2017.05.021
Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 2012;122(3):787-795. doi: https://doi.org/10.1172/JCI59643
Sodhi A, Tarang S, Kesherwani V. Concanavalin A induced expression of Toll-like receptors in murine peritoneal macrophages in vitro. Int. Immunopharmacol. 2007;7(4):454-463. doi: https://doi.org/10.1016/j.intimp.2006.11.014
Stunault MI, Bories G, Guinamard RR, Ivanov S. Metabolism plays a key role during macrophage activation. Mediators Inflamm. 2018;2018:2426138. doi: https://doi.org/10.1155/2018/2426138
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, Hoeksema MA, de Vos AF, de Winther MP. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684-696. doi: https://doi.org/10.1016/j.celrep.2016.09.008
Walia V, Kumar R, Mitra A. Lipopolysaccharide and Concanavalin A differentially induce the expression of immune response genes in caprine monocyte derived macrophages. Anim. Biotechnol. 2015;26(4):298-303. doi: https://doi.org/10.1080/10495398.2015.1013112
Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008. Chapter 14: Unit 14.1. doi: https://doi.org/10.1002/0471142735.im1401s83
Zhang Y, Morgan MJ, Chen K, Choksi S, Liu ZG. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood. 2012;119(12):2895-2905. doi: https://doi.org/10.1182/blood-2011-08-372383
Zhu L, Zhao Q, Yang T, Ding W, Zhao Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2015;34(1):82-100. doi: https://doi.org/10.3109/08830185.2014.969421
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 2, pp. 107-115, June, 2021
Rights and permissions
About this article
Cite this article
Zubkova, E.S., Dergilev, K.V., Beloglazova, I.B. et al. Features of the Population of Mouse Peritoneal Macrophages Isolated after Stimulation with Concanavalin A and Thioglycolate. Bull Exp Biol Med 171, 532–540 (2021). https://doi.org/10.1007/s10517-021-05265-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-021-05265-6