Abstract
Skin is the largest organ in the body, and directly contact with the external environment. Articles on the role of micro-current and skin have emerged in recent years. The function of micro-current is various, including introducing various drugs into the skin locally or throughout the body, stimulating skin wounds healing through various currents, suppressing pain caused by various diseases, and promoting blood circulation for postoperative muscle rehabilitation, etc. This article reviews these efforts. Compared with various physical and chemical medical therapies, micro-current stimulation provides a relatively safe, non-invasive therapy with few side effects, giving modern medicine a more suitable treatment option. At the same time, the cost of the electrical stimulation generating device is relatively low, which makes it have wider space to and more clinical application value. The current micro-current stimulation technology has become more and more mature, but there are still many problems in its research. The design of the experiment and the selection of the current parameters not standardized and rigorous. Now, clear regulations are needed to regulate this field. Micro-current skin therapy has become a robust, reliable, and well-structured system
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As the largest organ, the skin protects the human body and can receive external stimuli to provide feelings. In recent decades, there have been more and more research on skin irritation related to micro-current, which can be divided into the following four aspects, which is iontophoresis and electroporation for transdermal drug delivery, the promotion of skin wounds and chronic wound healing, transcutaneous electrical nerve stimulation (TENS) therapy for analgesia, and electrical muscle stimulation (EMS) therapy for postoperative rehabilitation and expanded applications.
In iontophoresis, direct current therapy is based on a reliable theory of electrical repulsion, and pulsed direct current has different waveform and duty cycles [1,2,3]. The delivery experiments carried out by researchers on various drugs and cosmetics have been very effective [4,5,6]. Electroporation therapy uses extremely high pulse voltage to introduce drugs into the skin. It has been widely used but takes certain risks. Its advantage is that it can transmit polymers [7,8,9]. Another application of electric current is to promote wound healing. When an external electric field acts on the human body's intrinsic electric field, it will affect the movement of cells involved in wound healing, thereby promoting the healing process, and the effect is very significant [10]. The research on chronic wounds is still lacking. As a safe non-invasive treatment, TENS therapy is divided into low-frequency TENS and high-frequency TENS clinically. It has a very obvious effect in inhibiting severe pain caused by many diseases. The principle of analgesia is to promote the secretion of analgesic substances and stimulate the sensory nerve in the body [11]. At present, TENS research still needs to standardize the experimental process and improve the system of pain evaluation. EMS was originally used for postoperative rehabilitation, and it has sufficient animal and clinical trial data to support it. Although there are still side effects during long-term treatment, it is far smaller than the previously mentioned current therapy [12]. In recent years, it has a wealth of applications in the game field, such as cooperating with virtual reality (VR) to create better game experience, improving sensory feedback mechanisms, and as an auxiliary device for sports [13].
This article will meticulously analyze the development and current status of various currents, as well as the future development direction. Compared to various physical and chemical medical therapies, micro-current stimulation provides a relatively safe, non-invasive therapy with few side effects. In the future, the skin's current stimulation will be more attractive, as a mature household device used by people to help people improve and enrich their lives.
2 Skin structure and stimulate function
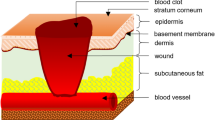
Skin is the largest organ in the body. It has a series of important functions such as protecting, regulating body temperature, excreting waste, and feeling stimuli. Anatomically, the skin is composed of three layers, namely the uppermost epidermal layer, the middle dermal layer, and the subcutaneous tissue, each of which functions differently [14]. The schematic diagram is shown in Fig. 1a [15].
a Structure of skin [15]. b Structure of the hair follicle: 1 – infundibulum; 2 – isthmus [16]. c Cross section of the hair follicle: Lanugo hairs are non- medullated and nonpigmented, present during intrauterine life [16]. d Protein levels of cytokines in BM-MSC-conditioned medium. (A) Antibody-based protein array analysis human dermal fibroblast (FB)- or BM-MSC-conditioned medium under hypoxic conditions. Similar results were obtained from three independent experiments and results from one of them are shown [18]. e Effect of BM-MSC-conditioned medium on wound closure. Representative images of wounds before treatment or 7 days after treatment with vehicle control medium (vehicle-M) (left), concentrated fibroblast (FB-M)- or BM-MSC-conditioned medium (MSC-M) (right) [18]
In recent decades, the formulations of transdermal drug patches have hardly increased, because the basic function of the skin is to protect and control the passage of substances, which hinders the delivery of drugs to the skin. Only a few drugs are suitable for molecular weight and transdermal absorption requirements, such as nitroglycerin for angina pectoris, clonidine for hypertension, and estradiol for estrogen deficiency. The transdermal drug delivery system (TDDs) may be the best new drug delivery system.
2.1 Skin structure
The epidermis is the superficial structure of the skin. The thickness of the epidermis is inconsistent throughout the body (0.06–0.8 mm), and plays an important barrier role. The outermost layer of the epidermal layer is the stratum corneum, and below it is the transparent layer, the granular layer, the spinous layer, and the base layer. The cell division at the base layer is active, and the continuously generated cells move upward from the granular layer to the stratum corneum, supplementing the aging and detached stratum corneum cells, undergoing keratinization and turning into dead cells without nucleus Lipid-containing adhesives stay together. On the other hand, as epidermal appendages, hair follicles are distributed on the entire body surface except the palms and sole. There are two types of hair, which is vellus hair and terminal hair. The terminal hair is thick, such as the hair of the scalp, eyebrows, eyelashes, and armpits, as shown in Fig. 1b, c, which is the structure of hair [16]. Each hair follicle consists of a hair shaft and a bulb. The ducts of the sebaceous and apocrine glands enter the hair follicle stem. Hair bulbs are composed of cells responsible for cell division, similar to the basal cells of the epidermis; then the cells differentiate to form an axis. The shaft is made of hard keratin. The mitotic rate of the hair matrix is greater than the mitotic rate of any other organ in the body. Hair growth is affected by any stress and disease that may alter mitotic activity. The effect of transdermal administration on vellus hair is negligible, but for terminal hair, the effect of mitosis must be considered. Although the blood vessels in the epidermal layer are very close to the skin surface, experiments show that the blood supply will not affect the epidermal penetration. The subcutaneous tissue is the hypodermis, which mainly supports the dermis and epidermis. The fat stored in it can regulate temperature and sense pressure, while protecting blood vessels and nerves.
2.2 Cells involved in wound healing
The skin contains numerous cells and tissues. Compared with the physical process of ion introduction, skin wound healing is spontaneous. Wound healing involves many types of cells, and it is a multi-party coordination and coordination process. When some of these factors are problematic, it can cause abnormal scars and chronic wounds. Adult stem cells (ASC) play a huge role in wound healing. Corneal epithelium healing and nerve regeneration involved in the repair process [17]. It is also a source of supplementary cells in wound healing. At the same time, tissue-specific ASCs also regulate other cells, such as inflammatory cells and tissue grandmother cells, to cooperate. Bone marrow derived mesenchymal stem cells (BM-MSC) have been shown to promote wound healing. Chen et al. [18] examined the paracrine factors released by BM-MSC and their effects on cells involved in wound healing, the antibody-based protein array analysis of fibroblasts or MSC-conditioned medium was performed under hypoxic conditions and normal oxygen conditions. As shown in Fig. 1d, fifteen cytokines with differential expression levels were found in 88 cytokines. Compared with normoxic conditions, hypoxic treatment significantly increases the release of several cytokines released by BM-MSC. The chemical attraction of MSC-conditioned medium and the promotion of mitotic wound healing requires the recruitment of cells from the surrounding tissues and blood into the wound. And then multiply. Wounds treated with vehicle medium or fibroblast conditioned medium were used as controls. Careful measurement of wounds on days 3, 7, 10, and 14 showed that MSC conditioned medium significantly accelerated wound closure compared to vehicle control medium or fibroblast conditioned medium, as shown in the Fig. 1e left, measurement of wound sizes is shown in the right of Fig. 1e [18, 19]. BM-MSCs releases high levels of cytokines and chemokines, which can enhance normal wound healing.
As key cells for wound healing, stem cells have different participants at different stages. Early injury is caused by inflammatory cell precursors from bone marrow and hair follicles, dermis and other locations, such as haematopoietic stem cells (HSC) from bone marrow (BM). Experiments in rats have shown that mast cell precursors in early wounds appear to be derived from BM and have been determined to respond to stem cell factor (SCF) [20]. These skin mast cell precursors and other haematopoietic progenitor cells (HPC) populations deposited in the dermis, then will participate in skin wound healing by supplying tissue macrophages and mature mast cells and dendritic cells Fig. 2d [21]. After this healing process is the re-epithelialization process, which also has multiple participants, of which keratinocytes come from two populations of skin epithelial stem cells (epiSC), which epiSC located in the base layer of interfollicular epidermis (IFE), hair follicle (HF) bulge epiSC in outer root sheath (ORS) Fig. 2c, the healing of the skin is not simply cell growth, but a variety of cells in the vicinity of the skin performing their duties, interconnected and coordinated work, and the regulation of this work is greatly affected by the electric field, which lays the foundation for the current treatment of skin wound foundation [21].
a Different routes of drug transport through skin [22]. b Percutaneous penetration of lipid vesicular carrier [22]. c Bulge region of a human hair follicle [21]. d Stem/progenitor cell populations that contribute to cutaneous wound healing [21]. e Cross-sectional view of polymer membrane permeation-controlled TDD systems [15]
2.3 Electrical stimulation on skin
The use of electric current to stimulate the skin is a large field of treatment in the medical field, and the results are quite fruitful, but there is not a clear enough distinction between the types and benefits of different micro-currents. Compared the benefits of current stimulation on skin, it can be divided into the following four aspects.
The research on transdermal current stimulation is the most extensive and thorough. The main therapies are iontophoresis and electroporation, and iontophoresis is common; Promotion of skin wound healing, which benefit has received increasing attention in recent years, and has gradually expanded to other fields, achieving attractive results; Transcutaneous electrical nerve stimulation for analgesia, with TENS percutaneous neurotherapy which is more often used as an adjuvant therapy and is combined with other therapies; The postoperative muscle recovery, using EMS electrical stimulation therapy, which is widely used in the postoperative recovery.
3 TDDs of micro-current stimulation
TDDs can be administered systemically or locally, which means the drug has penetrated through three layers enters the systemic circulation, or is applied topically only through the stratum corneum. Transdermal administration by passes liver metabolism, avoids the "peak and valley" effect of injection therapy.
There are four ways of transdermal administration, namely the transappendageal, transfolicular, transcellular, and intercellular routes. The different routes are shown in Fig. 2a [22]. Transcellular rout: Direct access to the dermis through keratinocytes and lipid bilayers, which includes active transport of ions, polar compounds, and endocytosis of macromolecules, which is the transport of molecules across epithelial cell membranes. Intercellular route: It is also called the paracellular route, which is a common way of drug molecular penetration. Cells are tightly connected by junctional adhesion molecules (JAMs). Closing proteins control the functioning of the barrier and select substances to transport between the cells. At this time, the drug stays in the lipid and is located around the keratin, so it is generally more suitable for the penetration of fat-soluble drugs. Transappendageal and transfolicular route: it is also called an appendage route, which includes sweat glands, hair follicles, and related sebaceous gland transportation. Although it avoids the stratum corneum, it only accounts for about 0.1% of the total skin area. The effect is secondary and is usually not considered in drug transport.
The path taken by drug penetration is mainly determined by the partition coefficient. The lipophilic molecules pass through the stratum corneum through a paracellular route. The hydrophilic molecules usually through transcellular route, but in fact the paracellular route is very tortuous, which greatly hinders the penetration of most drugs. The lipid vesicular system is another type of drug delivery method that can improve the bioavailability of encapsulated drug and extend the treatment time in a controlled manner. The transdermal penetration of lipid vesicle carriers is composed of physiological lipids. Vesicle carriers also destroy the lipid structure of stratum corneum, resulting in increased fluidity, thereby effectively transporting the carrier and drugs across the skin, increasing the transdermal permeation, as shown in Fig. 2b [22]. The penetration ability of drug nanocarriers (NCs) in biohydrogels will affect the efficiency of drug delivery [23]. It is worth mentioning that of the two main modes of transport, the transcellular route is relatively safe and easy to control. In addition to the nature of the molecule, the external environment can also affect transdermal administration. The stratum corneum of the skin is a great obstacle to transdermal administration, and it is composed of dead cells making it extremely resistant. Japanese scholars have studied the electrical properties of the stratum corneum skin, using cellophane to peel the stratum corneum layer by layer. With the peeling of the skin, the resistance decreases rapidly. When the number of strips reached 15 times, the change rate becomes constant, and the resistivity is approximately equal to the resistance of the subcutaneous tissue. Rate, that is, the part below the stratum corneum does not become a resistive layer [1].
At present, the medical community has used various methods, such as chemical accelerators, physical aids and delivery vehicles, to enhance the transdermal delivery of biologically active substances. The transdermal drug delivery (TDD) systems for polymer membrane penetration control, as shown in Fig. 2e, is a physical assistance method [15]. Allow the drug to penetrate through the rate control membrane. Electroosmosis therapy, as one of the physical auxiliary therapies, can reduce changes in drug plasma levels, and the effect is satisfied.
3.1 Iontophoresis
Iontophoresis is classified according to the flow of current. There are continuous direct current (CDC) and pulse depolarization iontophoresis (PDP). CDC is the earliest and common, but it will cause skin polarization and adverse irritation. PDP is a pulsed current that can alleviate the adverse effects and called depolarizing currents. Some literatures mention the use of alternating current (AC) for testing, which actually belong to pulsed direct current with direction change. In order to prevent confusion with the standard AC below, it is called a bidirectional pulse current.
The very important point of skin medication is that transdermal drug delivery is needed to achieve a therapeutic dose, which makes a long time, also the single application time, and it will be affected by sweating and other reasons. Transdermal patches can well solve the problem of long-term application. The development of "matrix" patches combines drugs, polymer films and adhesives to form a single "matrix" layer. This design allows them to be small and thin, and avoid the use of irritating substances. For example, Fig. 3a is a cross-section of a typical matrix patch, including a base film, a vial, a matrix, an adhesive, and a release paper [24]. In general, patches can be classified as reservoir systems, matrix systems without rate-control membranes or matrix systems with rate-control membranes, as shown in Fig. 3b [25]. The storage system consists of three main parts: drug storage, rate control membrane and adhesive. The drug reservoir contains drugs and excipients. The drug penetrates the skin through the membrane and the adhesive. In the matrix system, the drug is located in adhesive. The adhesive serves as the basis for the formulation and the adhesive. In addition, the size of the patch limits the amount of drug that can be contained, and it can only deliver low molecular weight (≤ 500 daltons) and high lipophilic drugs [26]. Because of the obvious advantages and disadvantages of transdermal drug delivery, researchers have invented iontophoresis to aid the drug delivery and make up for its shortcomings.
a Cross-section of a typical matrix patch, in this case the rivastigmine transdermal patch (Exelon® patch, Novartis Pharma AG, Basel, Switzerland) [24]. b Types of transdermal drug delivery systems [25]. c In the experimental group, heparin after pulse current was observed in both the stratum corneum and the epidermis, and fluorescence was also observed in some areas of the dermis. Total magnification × 100 (right). In the control group, no fluorescence was observed in the epidermal layer and the dermal layer. Total magnification × 100 (left) [28]. d Histology of rat skin treated or untreated with pulsed current iontophoresis. Rat skin treated by pulsed current iontophoresis, total magnification × 200 (left), control (right) [28]. e Iontophoresis using a Ag/AgCl electrode system [27]
Iontophoresis is a non-invasive therapy. It places a small charge in an electroosmosis room containing similar charges, inserts a wet pad between the electrode plate and the skin to prevent the skin from being burned, and the anode repels positively charged chemicals. While the cathode repels negatively charged chemicals, the influence between the electrodes along the skin surface is negligible, and it is essentially active transport. In addition to its electrical repulsion, it can also bring about an electroosmotic flowing effect. Physiologically, the skin is negatively charged with PH, and the cations are selective. The specific example is the Ag/AgCl electrode system, as shown in Fig. 3e, in the anode compartment, the pharmaceutical preparation (D+A−) containing the ionizable drug D+ is placed in the electrode compartment with the same charge. The electrode chamber is placed at the far end of the skin, and an electric potential is applied to cause a current to flow through the circuit to introduce the same charged ions into the skin [27]. Pacini et al. [28] used pulsed current electroosmosis to introduce fluorescently labeled heparin into the skin of living rats, as shown in Fig. 3c. In the pulsed current electroosmosis experimental group (left), fluorescence was observed in the stratum corneum and the epidermis. For the marked heparin, fluorescence can also be observed in some areas of the dermis, and the contour of the fluorescence is distributed along the intercellular path, and the contour of the cells can be clearly seen, but no fluorescence was observed in the epidermis and dermis in the control group (right). Pacini et al. [28] also studied the effect of iontophoresis on the skin. After staining the skin after the experiment, they found that there was no significant difference between the treatment group (left) and the control group (right), as shown in Fig. 3d. Observed and well-preserved, the epidermal thickness of the experimental group was uniform and uninterrupted, and various connective cells were intact around. Iontophoresis can use a lower current to improve the transdermal delivery of drugs in a short period of time.
Under the influence of the current, molecules are transported through the electrolyte solution to penetrate the biofilm. This means that the CDC method or PDP method can use 0.5 mA/cm2 or less current to the skin, which can significantly speed up the drug delivery to the therapeutic dose, and at the same time, the human body can also accept such current levels [29]. Marro et al. [4] conducted a test of suitable skin for experiment for iontophoresis, using mannitol as the introduced drug, and found that pig skin is the most suitable for electroosmosis, and the permeability depends on voltage and current density. Bhatia and Singh used pigskin experiments and introduced luteinizing hormone-releasing hormone (LHRH) with iontophoresis. The results showed a significant increase in flux. The experimenter also used this method to study anti-anxiety, anti-diabetes, and cardiovascular diseases such as systemic systemic drug delivery [5]. Mutalik et al. [30] then continued to develop membrane-regulating systems for glipizide and matrix patch systems for the glipizide delivery, which was more effective than oral formulations, and showed that transdermal delivery of drugs using a combination of a drug and a polymer patch had good results and successfully prevented lower in the first few hours glucose and is also effective for chronic applications. Compared with oral administration, the stimulating effect of the transdermal route on the skin is negligible. The scanning electron microscope of the preparation patch showed that after in vitro penetration studies, pores were formed on the surface, as shown in Fig. 4a, the smooth surface of the film (A and B) penetrated and the surface became rough (A1 And B1).
a SEM photographs of transdermal patches A: EC/PVP (3:2) before permeation studies; A1: EC/PVP (3:2) after permeation studies; B: ERL/ERS (4:1) before permeation studies; B1: ERL/ERS (4:1) after permeation studies [30]. b Comparison of transdermal iontophoretic skin permeation profiles of LHRH by three different application pattems with a current intensity of 0.6 mA [3]. c Schematic illustration of different concentration gradients across skin during various durations of current application. The transdermal iontophoretic skin permeation of LHRH is proportional to the concentration gradient, whose equilibrium concentration gradient is time-dependent [3]. d Current (filled circle) and voltage (open square) profiles 1 h after the initiation of lysine iontophoresis with 50% square-wave, unipolar, pulsed DC at frequencies of (up) 2500 Hz, (down) 250 Hz [31]
Regard to the effect of the parameters of iontophoresis on the efficacy, the current research is mature. According to Faraday's law, no matter whether it is a pulse current or a stationary current, the ion throughput is equal within the time when the average current is equal. What needs to pay attention, is the current should not be too large, so you need to increase the current intensity while making the skin tolerate. When the voltage is the same, decreasing the skin impedance increases the current. Plutohik pointed out that when the frequency is increased from 1 to 100 Hz, skin impedance will decrease from 130 kΩ to 30 kΩ [2]. For pulse current therapy, square wave pulses and exponential pulses are common. It is generally believed that square wave pulses can significantly increase drug permeability by increasing energy before reaching the energy saturation value. For exponential pulses, when the electroporation energy is unchanged, the transdermal rate of the drug will decrease over time. For AC pulsed electroosmosis therapy, Yamamoto found that when the frequency of the pulsed current is increased, the impedance on both sides of the skin decreases and the effective intensity on both sides increases when the current intensity is constant [1]. Chen and Chien [3] performed a pulsed direct current electric iontophoresis experiment of LHRH on the skin of hairless rats, using different waveform and duty cycles. The results show that the pulse waveform has little effect on penetration enhancement. However, the triangular wave has the smallest increase, and the higher the duty cycle, the more significant the enhancement of the penetration, the researchers used a polyacrylamide hydrogel storage device to characterize the effects of several electrical parameters on the transdermal iontophoresis of LHRH hairless rat skin. When current is applied continuously instead of in between, LHRH has a higher iontophoretic permeability. As shown in Fig. 4b, when direct current (DC) of 0.6 mA is continuously applied for 3 h, the total accumulation of LHRH in current application mode A is the highest, and the LHRH iontophoresis in mode C is the least. The schematic diagram of different concentration gradients on the skin during different durations of application is shown in Fig. 4c. LHRH transdermal iontophoresis skin penetration is proportional to the concentration gradient. The equilibrium concentration gradient is related to time. The difference between the various waveforms is not obvious. The on–off rate of pulsed direct current has a significant effect on the transdermal iontophoresis of LHRH, and proving that the higher the on–off rate (duty cycle), the greater the skin permeability of LHRH. For percutaneous iontophoresis, the duration of current application is more important than the amplitude and intensity of the current. It is worth noting that Hirvonen et al. [31] had the amino acid iontophoresis experiments show that constant direct current electroosmosis therapy has the highest efficiency under the same charge, and pulse electroosmosis cannot provide drugs that exceed the CDC current, and the frequency of the pulse does not significantly affect the delivery, as shown in Fig. 4d. It is a waveform of a unipolar square wave with a 50% duty cycle at different input frequencies. When the frequency increases, the voltage lags behind the current, witch due to the skin changes caused by electric current. The polarization of skin caused by osmotic therapy inevitably reduces its efficiency over time. Cazares-Delgadillo et al. [6] used different types of currents (DC, square unipolar pulse current (SPC), DC + pulse current (PC)) to perform skin experiments on cosmetics (ascorbic acid (AA) and ellagic acid (EA)). The current waveform is shown in Fig. 5c, d, when different percentages of AA solution were used, the import results are shown in the Fig. 5a, the group with a high AA content had more deposits, and the DC + PC group had the best effect. The DC group was second, and the SPC group had no advantage over the control group. In addition, the pH of the solution is also a factor that affects the amount of deposition in a certain period of time. As shown in the Fig. 5b, when the EA solution pH is 7, the EA molecules are mainly negatively charged, which increases compared to the amount of pH 4 [6].
a Deposited amount of AA obtained with AA Formula 1 (5%) and AA Formula 2 (10%) at each current profile (0.2 mA/cm2) for 5 min iontophoresis (n = 6) [6]. b Comparison of the deposition of 0.2% EA solution at pH 4.0 and pH 7.0 at 0.3 mA/cm2, applied for 10 min and 20 min iontophoresis. (l) Scanning electron microscope image of flufenamic acid-loaded FA-PLGA nanoparticles [6]. c Negative polarity current profiles, galvanic DC [6]. d Negative polarity current profiles, from left to right are galvanic direct current and pulse current (DC + PC), and SPC [6]. e Scanning electron microscope image of flufenamic acid-loaded FA-PLGA nanoparticles [44]. f Effect of lidocaine on porcine epidermis. Light micrograph of a 1 μm thick plastic section showing alteration of pig epidermis produced by iontophoresis of 4% aqueous lidocaine hydrochloride (3 mA for 45 min) in vivo. Note the dense cytoplasmic staining, the darkly stained flattened nuclei in the upper stratum spinosum and the stratum granulosum and the vacuolation of cells of the basal layer [32]. g Light micrograph showing control porcine skin [32]
The principle and efficacy of iontophoresis have been widely recognized, so safety and drug delivery toxicity are worthy of attention. Studies have shown that the rate of reduction in skin resistance depends on the applied voltage, and the time for reversible recovery depends on the size and duration of the electric field. The skin irritation in iontophoresis is partly due to the polarization caused by continuous DC induction. By applying current in a high-frequency pulse waveform, especially by briefly reversing the current during each cycle, this polarization can be avoided. Studies have shown that when lidocaine is released from sodium chloride aqueous solution for 10, 40 or 100 min within a certain current range, various macroscopic and microscopic changes occur in the pig epidermis, as shown in the Fig. 5f, g, vacuolation of basal layer cells occurs. Clinically, it is easy to observe various degrees of erythema and "petechia". The erythema below the anode usually disappears after 24 h, while it is very rare under the cathode. The cathode is associated with a higher degree of edema and the formation of some blisters [32]. Okabe et al. [33] carried out a study in volunteers and used beta-blockers for transdermal experiments, and no skin damage has been observed. The effects of transdermal administration are divided into two types: local delivery and systemic delivery. Other researchers have tried using electroosmosis to treat arrhythmias. They delivered an effective dose of verapamil, and concluded that electroosmosis would be safer and more reliable if delivered with high systemic toxicity [34]. A recent study shows that electrical stimulation can control the rate of peptide release, especially fibrin peptides used for tumor image diagnosis and inhibition of tumor cell migration, displaying a wide space for electrical stimulation therapy [35]. In fact, the PDP method is the mature therapy in iontophoresis, and local delivery greatly reduces adverse reactions such as skin polarization and redness, and systemic delivery is better than oral and injection. Iontophoresis is very compatible with other therapies and works well. Wangetal researched the efficacy of iontophoresis combined with the microneedle therapy, ultrasonic dialysis therapy, and chemotherapeutic therapy, and believed that combined therapy can increase the poor water solubility and the penetration efficiency of macromolecular compounds [36]. It is concluded that the combination of very low density current with penetration enhancers can significantly improve efficiency, and with this current density, the skin resistance can be restored as before. Nair et al. [37] studied the penetration of tartrate to enhance the penetration of chemical penetration enhancers combined with iontophoresis. Donohoe et al. [38] conducted vagus nerve stimulation (VNS) experiments with electrical impulses, which can alleviate seizures such as epilepsy, which is an advancement of existing therapeutic nerve stimulation. Oh and George [39] used conductive polymers for drug delivery to increase the nerve recovery after stroke. Wang et al. [40] designed a nanogenerator system to assist in the proliferation of osteoblasts, and proved the plasticity of P (VDF-TrFE) piezoelectric nanofiber NG, which can be used in a self-powered electrical stimulation system, and proposed its use in tissue and important potential applications in regeneration. Sebastian et al. [41] studied iontophoresis and lactic acid combined therapy, and pointed out that the ionic properties of the penetration enhancer molecules will affect the combined therapy. Bottoni [42] pointed out that although the use of silver ions and micro-current can reduce the number of bacteria in vitro, it is not clear that whether they can effectively reduce postoperative infections. Further research should be done when making micro-current silver electrodes. Mousavi et al. [43] developed a silk fibroin film-based drug delivery device and made it conductive, which can be electrochemically loaded, which is a new application of electrical stimulation. In addition, Gomez et al. [44] encapsulated flufenamic acid with negatively charged nanoparticles and introduced it into the body using iontophoresis. Figure 5e shows the negatively charged nanoparticles with FFA, although the results show that the penetration promoting effect is not ideal, but this shows the research potential of iontophoresis. Kolli et al. [47]. Electroporation also has two types of exponentially decaying waves and square waves, and most cases are exponentially decaying waves. Electroporation is a new drug delivery method for macromolecular substances. Human skin is complex, with many functions and features, and candidate sites with multiple pathways are shown in Fig. 6d. A "brick wall model" is introduced to describe the passive diffusion of aliphatic molecules on SC. The lipid-based structure tends to exclude charged substances, the cell membrane of SC, the cell layer connected by tight junctions, and the multi-layer lipid bilayer membrane of SC all has relatively large electrical resistance, and electroporation occurs at a higher voltage, formation of water channels and electrical drive transmission of small ions and water-soluble molecules [48] voltage is usually several hundred volts, and the time constant is about 5 ms. Researchers have pointed out that in addition to short-term high-voltage pulses that cause electroporation, electroporation can also occur in conventional iontophoresis (when low voltage is applied for a long time), and analyzed the relationship between the location of electroporation and the electric field. As shown in Fig. 7d, when the induced electric field strength E = ET, the minimum transmembrane electric field strength required to cause dielectric breakdown. When E > ET, other electroporation is induced at a farther distance. If the motion is directed at the transmembrane electric field, the net negatively charged entities inside the spherical membrane will diffuse through the pores. The net positively charged entities inside the spherical membrane can diffuse out because the direction of motion is the same as the electric field near the hole [49].
a Schematic of the stratum corneum with LDR, a localized region (radius = 30–500 μm) where electric energy is dissipated, and which is characterized by a drop in resistance [47]. b A complete set of results from one experiment. The pulse parameters were Uskin = 83 V and τpulse = 95 ms [47]. c Model for the electrothermally induced changes at stratum corneum. (A) Stratum corneum before high-voltage pulse, (B) during electric current flow with propagating heat front starting at the center of maximal current density, and (C) partial recovery of the stratum corneum after pulse at sites where the phase transition of the lipid structure was not reached [47]. d Key features of skin, skin barriers and hypothetical aqueous pathways [48]
a Photograph of SKH-1 hairless mouse being treated with parallel plate electrode under isoflurane inhalation anesthesia. (Inset) Close-up of one of the plates of parallel plate electrode showing it recessed by 0.5 mm to allow a space for a conductive agar gel to be placed on it [7]. b Typical response of a melanoma to three applications of 100 pulses (300 ns, 40 kV/cm, 0.5 Hz) 30 min apart on day 0 followed by a single application on day 4 using a 5 mm diameter parallel plate electrode on mouse #102. Collection of seven matched sets of images of the same tumor all taken on the day indicated in the lower left corner of the transillumination image [7]. c Blood flow in melanoma before and after nsPEF application. (A) 3-D reconstruction of volume of melanoma; (B) power Doppler reconstruction of blood flow before field application. (C) 3-D reconstruction of volume of the same melanoma shown in (A) generated about 15 min after 100 pulses (300 ns, 40 kV/cm, 0.5 Hz). (D) Power Doppler reconstruction of blood flow in the same tumor shown in (B) generated about 15 min after 100 pulses (300 ns, 40 kV/cm, 0.5 Hz) [7]. d Location of electropores with respect to electric field. (A) One electropore at pole of each hemisphere when induced electric field strength, E, is equal to the minimum transmembrane electric field strength needed to cause dielectric breakdown, ET. (B) Additional electropores induced at greater distance from poles when E > ET. (C) Net negative charged entity inside spherical membrane is inhibited from diffusing through electropores if movement is against transmembrane electric field. (D) Net positive charged entity inside spherical membrane can diffuse out since movement is in same direction as electric field in vicinity of pore [49]. e Photographs of formalin-fixed, paraffin-embedded cross-sections of porcine buccal mucosae. Hematoxylin and eosin staining: (A) Control, untreated. (B) Control, passive permeation after an 8 h treatment period. Slightly enlargement of 2–3 cell layers (arrows) [50]
Electroporation can be used in combination with many other technologies and has a good effect. For example, electroporation can be combined with ultrasound or iontophoresis. The introduction of luteinizing hormone release hormone has a five-fold increase in permeability compared with the use alone, and reduces skin erythema, the same composite technology also has a good promotion effect on macromolecular drugs such as insulin and calmodulin [9].
High-frequency direct current may cause skin damage, causing erythema, herpes, and even burns. Jacobsen's in vitro three-chamber osmosis cell promoted atenolol hydrochloride ion penetration experiments showed that the outer layer of epithelial cells was disturbed after a treatment period of 1 mA at 8 h, as shown in Fig. 7) [50]. Compared with skin redness and swelling caused by CDC or PDP therapy, the effect of electroporation is relatively slight, which can cause focal intraepidermal edema and vacuoles, which increased with increasing voltage. However, for electroporation, skin redness, burns, fever, nerve irritation, and irreversible electroporation are clearly pointed out, and dose prediction between different drugs, the model is not universal and the prediction is more complicated [26]. Jacobsen [50] found that electroporation can treat skin tumors and cause the tumor cell nucleus to contract quickly and the tumor blood flow to stop. The hairless mice clamped by the parallel plate electrodes are shown in Fig. 7a, and the electrode diameter is 3–5 mm. These electrodes are coated with conductive agar to separate the skin from the electrodes. The distance between the two plates is 0.5–1 mm, the number of pulses is 100, the duration is 300 ns, the amplitude is 4–8 kV, and the frequency is 0.5 Hz. As shown in Fig. 7b, there was a difference between the treatment group and the control group two days after the experiment. The black SC appears on the stratum corneum of the pulse area and regenerates in the stratum corneum. It consists of coagulated red blood cells. After treatment, the tumor usually shrinks by 90% within two weeks. Figure 7c is a 3-D reconstruction of the volume of melanoma. Histology confirmed that red blood cells scatter in and around the melanoma tumor. This means that local blood vessels leak and red blood cells escape into the surrounding tissue. Tumor blood flow usually cannot be restored [7].
Iontophoresis and electroporation are widely used as two important therapies for transdermal introduction. Iontophoresis is mainly applied to drugs, and the movement of charged particles caused by electrostatic repulsion will incidentally change the structure of the skin, while electroporation changes the permeability of tissues, thereby increasing drug transport, regardless of the voltage applied electrode. Studies have shown that, from the applied voltage, electroporation contains ultrashort pulses with a duration of several milliseconds, with an intensity of several hundred volts, and a transdermal iontophoretic voltage of 3–12 V, which lasts from several minutes to several hours [51]. In effect, the mechanism of electroporation and iontophoresis is still controversial, such as Weaver believes that the difference lies in whether new channels are created, but from the foregoing, it can be seen that with low voltage for a long time similar pores also appear in the electroosmotic therapy, and Hui believes that both use the electric field to increase the permeability of skin accessory channels, there is no essential difference [52]. It is not accurate at all. Electroporation is obviously different from iontophoresis from the application mechanism to the effect, but there is an overlap in the phenomenon. Overall, these two therapies are now relatively mature.
4 Micro-current stimulation for wound healing
Human skin is the largest organ exposed, and injuries are very common. In addition, there are many diseases that can cause difficult-to-heal wounds on the skin, such as venous ulcers, which keeps the body in a morbid state and will cost huge medical expenses. The principle of wound healing is more complicated. The healing process consists of four stages (in order): the formation of temporary wound matrix (including hemostasis and coagulation), the accumulation of neutrophils and monocytes, the formation of granulation tissue, re-epithelial tissue and blood vessels Network recovery (proliferation and repair), resha** phase. The first stage of wound healing is hemostasis and the formation of a temporary wound matrix. This process occurs immediately after injury. As shown in Fig. 8a, the wound healing inflammation stage is activated during the hemostasis and coagulation stages and can be roughly divided into neutrophil recruitment stage of monocytes and the late stage of monocyte appearance and transformation. The next step is the proliferative phase. The final step of the proliferation phase is the development of acute granulation tissue. As shown in Fig. 8b, the remodeling phase has begun. As a transitional tissue, it replaces the fibrin-based fibronectin-based temporary wound matrix and may cause scarring as it matures. Finally, during the remodeling phase, the formation of granulation tissue is stopped by apoptosis. During wound maturation, collagen III produced in the hyperplastic stage is now replaced by stronger collagen I. As shown in Fig. 8c, this type of collagen is oriented into small parallel bundles, and myofibroblasts pass through multiple attachments cause wound contraction and help reduce the surface of the scar that is forming [53]. There is a clear understanding of the mechanism of wound healing, but related therapies are not systematic. Modern medicine has greatly developed treatment of wounds. Therapy involves many aspects.
a Inflammatory phase after a cutaneous cut; hemostasis and invasion of inflammatory cells [53]. b Proliferative phase; organization of the thrombus, secretion of growth factors, synthesis of collagen III and the beginning of angiogenesis [53]. c Remodeling phase; regenerative processes fade and are followed by reorganization of the connective tissue and contractile response [53]. d Generation of skin wound electric fields. When wounded, the potential drives current flow through the newly formed low resistance pathway generating an electric field whose negative vector points toward the wound center at the lower portion of the epidermis and away from the wound on the upper portion below the stratum corneum [57]
4.1 Wound healing
The electric field plays a very important role in skin wound healing, and it can guide cells to complete the healing work naturally and orderly [10]. The microenvironment in the human body has a moderating effect on the biological behavior of cells [54]. At the same time, to study the specific impact of the microenvironment on cells, it is necessary to observe the microenvironment, such as the local oxygen microenvironment, which requires the assistance of materials such as hydrogel [55]. In both physiological and pathological conditions, cells can be observed to migrate in the interstices of tissues, including immune cells and cancer cells. Electrical stimulation can also regulate cell movement by promoting the formation of intercellular pores [57]. c The diagram illustrates the interaction between electric fields. The electric field can be directly attached to the endogenous electric field of the wound, where applying EF (brown arrow) in the default direction of wound healing will enhance wound-induced endogenous EF (red arrow), thus increasing the wound healing rate. In contrast, reversing the direction of EF (blue arrow) against the default wound healing direction will suppress the endogenous EF in the wound (red arrow), thus reducing or even stop the normal wound healing behaviors [10]. d Directional angiogenesis is a vascular-like structure of a rat placed in a 3D armature chamber with the aortic ring growing toward the anode. On the left is the aortic ring after embedding; on the right is day 3, after 200 mV/mm EF treatment. Rod is 500 μm [10]
The rat experiments of Long et al. [58] show that electric fields can promote fibroblasts, to differentiate muscle cells and provide contractile force to close the wound. When fibroblasts are cultured separately, with 6 h electric field stimulation, fibroblasts can be observed to differentiate along the electric field lines. As shown in the Fig. 10d. Zhao et al. [59] also tested the effect of electrical signals on cell movement. The researchers observed cell migration in a single layer of epithelium. When using a polarity opposite to the default treatment direction, epithelial cells followed the electrical signal movement, the wound opened, and the polarity was reversed. After sexual wounds are closed, as shown in Fig. 10a, similarly, corneal cells move faster when the treatment electric field is applied, and wound healing speeds up, but when the opposite polarity electrode is applied, it will hinder healing, as shown in Fig. 10b, c. Another study by Zhao [60] explained the specific principle of how electric fields affect cells. The researcher fluorescent cells such as F-actin (which drives cell movement) and placed them in the electric field. The redistribution of fluorescent markers in keratinocytes and corneal epithelial cells was observed within minutes. As shown in Fig. 10e, the polarized agonist polymerized to cause the cells to move directionally, that is, the cells were depolarized on the cathode-facing side and hyperpolarized on one side of the anode. At the same time, study have pointed out that multi-cell behavior is more complicated than single cell behavior. The behavior of collective cells involves cell polarization, and this process is also regulated by electrical stimulation [61]. Embryonic stem cells differentiate in many directions, and electrical stimulation can induce cell differentiation [62]. The mobility of different types of cells varies greatly. In vivo and in vitro experiments have also proven that it induces epithelialization and promotes wound contractions. Positive polarity will enhance the migration of epithelial cells and cause the wound to close faster. Using the cathode as the active electrode can more effectively attract fibroblasts to the wound area. Figure 10f from left to the right is the control group, the cathode ES group and the anode ES group, 7 days after the experiment, the number of fibroblasts (F) in the cathode ES group was significantly higher than that in the control group, and more collagen could be produced. In addition, electrical stimulation can promote fibroblast differentiation [63]. Skin fibroblasts exposed to 50 mV/mm contracted the collagen gel matrix. After exposure to ES, fibroblasts added to type I collagen gel and cultured. The diameter of each collagen gel was measured at 48 h and 72 h. As shown in Fig. 11f, the experimental group contracted significantly. Another experiment revealed the effect of ES on the expression of α-SMA in fibroblasts. Skin fibroblasts were seeded on conductive membranes and exposed to different times of 50 or 200 mV/mm. Then, the cells were separated from the conductive membrane, washed, and seeded on a cover glass. Myoblasts labeled with cytoplasmic immunity were proposed. As shown in Fig. 11d, the number of labels increased significantly [64]. In addition, electrical stimulation can cause an increase in capillaries. ES has been shown to increase local blood circulation, increase tissue perfusion, and promote capillary growth. Recent studies have shown that electrical stimulation can lead to enhanced expression of neuroendocrine markers in the wound and can enlarge innervation. Studies by Sebastian et al. [65] showed that neuropeptide synthesis is up-regulated during ES treatment, as the treatment time increases, PGP9.5+ nerve fibers gradually increase in healing wounds Fig. 11b, especially on the 14th day after treatment, on the other hand, SP (substance P, neurotransmitter) expression increased under ES treatment Fig. 11a. Besides, for the first time, Lallyett et al. [66] conduct an analysis of the efficacy of electrical stimulation at the genetic level. The results of the study showed that a specific ES would cause the differential expression of 105 genes, and most of them were down-regulated.
a An EF directs migration of corneal epithelial cells in a monolayer model of wound healing (150 mV/mm) [59]. b Stratified corneal epithelium migrate in situ to heal a wound (towards the left) [59]. c Electric fields applied with polarity opposite to the default healing direction direct the wound edge to migrate away from the wound [59]. d Electrical stimulation experiments of fibroblasts cultured in the absence of (control) and (experimental) electric fields. It can be observed that fibroblasts differentiate along the electric field line [59]. e Polarized EGF receptors, dp-ERK (activated mitogen-activated protein kinase) and actin polymerization. Dp-ERK1/2 and F-actin are surface plots that represent fluorescence intensity [60]. f Light micrograph (hematoxylin and eosin, 100) of full-thickness wound in guinea pig 7 day after incision, control, cathodal ES, and anodal ES, respectively, from left to right. Cathodal and anodal DC ES (sensory intensity) was applied for 1 h per day, every other day, for 7 days. The number of fibroblasts (dark fusiform cells, F) was significantly higher in cathodal ES group compared with control group [63]
a Representative immunohistochemistry (IHC) of SP expression. Dotted lines indicate epidermal-dermal junction. Scale bar is 100 μm [65]. b IHC of PGP9.5 expression [65]. c Confocal microscopy imaging (A and A’) of colocalization of E-cadherin and vimentin-positive cells at day 6 after injury; insets provide its higher magnification [72]. d Effect of ES on fibroblast a-SMA expression. Dermal fibroblasts were seeded on conductive PPy/HE/PLLA membranes followed by exposure to 50 or 200 mV/mm for various periods. The cells were then detached from the conductive mesgmbranes, washed, seeded on coverslips, and cultured up to 70% confluence. The cells were then stained using relevant monoclonal antibodies. Cytoplasmically immunolabelled myofibroblasts are presented [64]. e A constant direct current. B double peak unidirectional current, that is, high-voltage pulse current [HPVC] or intermittent low-intensity direct current. C proves double-symmetric current, also called low-voltage pulse current. D biophasic symmetrical, which is a TENS. E balanced asymmetrical biophasic [69]. f Dermal fibroblasts exposed to 200 mV/mm highly contracted the collagen gel matrix. Following exposure to ES, the fibroblasts were added to a collagen type I gel and cultured. The diameter of each collagen gel was measured at 48 h and 72 h. Representative photos show the action of the ES-exposed and non- exposed cells on collagen gel contraction [64]. g Skin blood flow in a typical subject measured by the laser doppler flow imager before treatment (a), after 20 min wound heating (b) and just at the end of electrical stimulation (c). The whiter the color, the greater the blood flow. Blue represents the lowest blood flow [71]
4.2 Chronic wound
Chronic wounds are defined as wounds that have not been repaired after 3 months of appearance. Any disease may cause chronic wounds, and the cause is usually referred to as a reference therapy. Common causes of chronic wounds, such as neurological diseases, hemolytic anemia, rheumatoid arthritis, insufficient arterial blood supply, etc., are mostly related to blood problems [67]. Nair compiles clinical experimental studies on the treatment of chronic wounds by micro-current. The mechanism of action is the triggering or promoting effect of the healing process, and it has a more significant effect as an auxiliary therapy and has great potential [68]. Research on the healing of chronic wounds involves a wide variety of currents, including low-intensity direct current, low-voltage pulse current, alternating current, and transcutaneous TENS, as shown in Fig. 11e, and low-intensity direct current is the first therapeutic current used was tested in both animals and humans [69].
Gault et al. [70] used a constant low-intensity direct current (200–800 μA) to treat patients with ischemic skin ulcers. The researcher applied the negative electrode to the wound three days ago to play an antibacterial and clear necrotic role. After the wound are cleaned out, the positive electrode for the electrode was inverted the average healing time, after 2-h treatments three times a day for 4.7 weeks. The important cause of chronic wound formation is insufficient tissue oxygen supply and insufficient nutrition caused by poor blood flow. High frequency pulse current can greatly alleviate this situation. Petrofsky et al. [71] treated patients with foot ulcers, using ES (two-way sinusoidal current 250 μs, 30 Hz, 20 mA) and local dry heat therapy combined experiment, compared with a control using dry heat therapy after one month of treatment, the reduction of the average wound area in the experimental group was twice that of the experimental group, Fig. 11g shows the skin blood flow of a typical subject measured with a laser Doppler blood flow imager before treatment (a), after 20 min of wound stimulation (b), and at the end of electrical stimulation (c). The whiter the color means greater the blood flow. Blue represents the lowest blood flow. The results showed that the blood flow to the wound and the skin around the wound increased with the increase of ES. Cramp et al. [72] designed high-voltage pulsed electrical stimulation experiments showed that the use of electroporation to remove parts of the skin can be regenerated without scarring, and this method has few side effects, and at the same time, p63 (regulating the proliferation and differentiation factors of mature keratinocytes) had a significant expression after 1 day of electroporation Fig. 11c, the size of the cells marked by staining changed (pointed by the black arrow), and showed on the basement membrane migration phenomenon, the expression of p63 increased to a basic level after one week.
Research on biocompatible conductive materials is also a hot spot in recent years. The review by Huang et al. [73] pointed out that the cell microenvironment has become a key factor in determining the cell growth. The corresponding development of bionic materials is an important source of triggering and regulating cell behavior. Zhao et al. [74] have manufactured bio-materials with high electrical conductivity, wrapped in nano-layers, and can be widely used in the fields of cardiac tissue engineering and flexible electronic devices. Liu et al. [75] designed the electrostimulation wound dressing cloth by making non-proof cotton coated with silver oxide film and the zinc coating, which can significantly accelerate wound healing process, which has strong antibacterial ability and good cell compatibility. Studies have also show that intrinsically conducting polymers (CPs) show great advantages in promoting wound healing, can solve the problem of electrical stimulation applied to the skin, and can be combined with polymers. CPs has great potential in flexible wearable electrical stimulation therapy [76]. Kai et al. [77] use flexible enzyme electrodes and stretchable hydrogels to make bioelectric plasters with good compatibility. Experiments in mice prove that bioelectric plasters can accelerate wound healing. Qing et al. [78] developed a new type of heterostructure composite scaffold, composed of electrospun nanofibers with high conductivity and good biocompatibility on graphene-coated paper, which can induce and enhance the directional growth of neurons, providing graphene. This direction has great potential in the field of stem cell therapy. Lu et al. [79] studied polyHEMA/PPY hydrogel, which can be applied to the skin, and is stronger than ordinary ES electrodes in terms of antibacterial and enhanced wound healing, and can cover and stimulate the entire wound area. This conductive hydrogel has great advantages in the treatment of chronic wounds. The application of biomimetic materials currently covers tissue, cells, genes and other levels, with great potential, and compared to other tissues, skin is more suitable for conducting electrical stimulation experiments with conductive polymers.
At present, experiments on electrical stimulation to promote skin wound healing have achieved good results, but only in the field of low-voltage direct current has a sound and clear theoretical basis and research progress. Other types of current are mostly used to treat chronic diseases caused by various reasons. However, due to the complexity of the etiology and the variety of currents, the voltage and current parameters in this part of the study are not clear enough, and the experimental results are not convincing enough. Current research on current stimulation of chronic wounds requires authoritative experimental data with clear parameters.
Today, 20 years later, this problem has not been solved. Although the experimenters have gradually realized the possible side effects of the current, the analysis of the experimental results is far from rigorous. This makes the field of current stimulation to promote wound healing. Research is not clear enough.