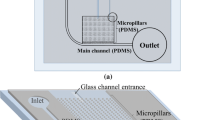
Abstract
Microfluidic analytical devices manufactured on paper and similar inexpensive substrates (µ-PADs) have shown considerable promise for disease diagnostics in resource-limited regions. However, current commercialization approaches can be improved substantially by addressing existing technical challenges associated with µ-PADs. Among these, off-device plasma separation from whole blood is a critical challenge in µ-PAD technology that limits commercialization. Existing µ-PADs made by combining multiple components require extra fabrication steps and manufacturing material. Our approach utilizes a two-step plasma process to fabricate single-layer semi-enclosed µ-PADs directly on a commercially available blood plasma separation membrane to incorporate blood plasma separation functionality into the device. The semi-enclosed µ-PADs are bonded with low-cost adhesive plastic tape to provide mechanical support to the device and make it more mechanically robust for field applications. Detection zones of the µ-PADs have also been modified with a cellulose nanocrystal (CNC) to increase colorimetric signal homogeneity, thus enhancing signal quality. The CNC-modified µ-PADs have been used for colorimetric detection of two model analytes (glucose and albumin) in whole blood. Colorimetric signals for both glucose and albumin from whole blood samples were consistent with the calibration curves generated using stock solutions.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abe K, Suzuki K, Citterio D (2008) Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal Chem 80:6928–6934
Akyazi T, Basabe-Desmonts L, Benito-Lopez F (2018) Review on microfluidic paper-based analytical devices towards commercialisation. Anal Chim Acta 1001:1–17. https://doi.org/10.1016/j.aca.2017.11.010
Arvand M, Arjmandi N, Shakibaie M, Jafarinejad S, Shahghadami R, Sasanpour P (2020) Colorimetric microfluidic paper-based sensor for determination of nitrite in drinking water with enhanced color development. J Phys D: Appl Phys 53:355403
Balu B, Breedveld V, Hess DW (2008) Fabrication of “roll-off” and “sticky” superhydrophobic cellulose surfaces via plasma processing. Langmuir 24:4785–4790
Carrilho E, Martinez AW, Whitesides GM (2009) Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem 81:7091–7095
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS (2014) Recent developments in paper-based microfluidic devices. Anal Chem 87:19–41
Catunda AGV et al (2013) Blood leptin, insulin and glucose concentrations in hair sheep raised in a tropical climate. Small Rumin Res 114:272–279. https://doi.org/10.1016/j.smallrumres.2013.07.008
Coltro WKT, Cheng CM, Carrilho E, de Jesus DP (2014) Recent advances in low-cost microfluidic platforms for diagnostic applications. Electrophoresis 35:2309–2324. https://doi.org/10.1002/elps.201400006
Cripps A, Husband A, Scicchitano R, Sheldrake R (1985) Quantitation of sheep IgG1, IgG2, IgA, IgM and albumin by radioimmunoassay. Vet Immunol Immunopathol 8:137–147
de Tarso GP, Garcia Cardoso TM, Garcia CD, Carrilho E, Tomazelli Coltro WK (2014) A handheld stam** process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays RSC. Advances 4:37637–37644. https://doi.org/10.1039/C4RA07112C
Dungchai W, Chailapakul O, Henry CS (2011) A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 136:77–82
Evans E, Gabriel EFM, Coltro WKT, Garcia CD (2014) Rational selection of substrates to improve color intensity and uniformity on microfluidic paper-based analytical devices. Analyst 139:2127–2132
Gabriel EFM, Garcia PT, Cardoso TMG, Lopes FM, Martins FT, Coltro WKT (2016) Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst 141:4749–4756. https://doi.org/10.1039/C6AN00430J
Hochleitner K (2020) Diagnostic assays: how depth filters work in blood separation. https://www.cytivalifesciences.com/en/us/solutions/lab-filtration/knowledge-center/diagnostic-assays-blood-separation-using-depth-filters. Accessed 23 Jan 2021
Kao P-K, Hsu C-C (2014) One-step rapid fabrication of paper-based microfluidic devices using fluorocarbon plasma polymerization. Microfluid Nanofluid 16:811–818
Kar S, Maiti TK, Chakraborty S (2015) Capillarity-driven blood plasma separation on paper-based devices. Analyst 140:6473–6476. https://doi.org/10.1039/c5an00849b
Krentsel E, Fusselman S, Yasuda H, Yasuda T, Miyama M (1994) Penetration of plasma surface modification. II. CF4 and C2F4 low-temperature cascade arc torch. J Polym Sci Part A: Polym Chem 32:1839–1845
Lewis GG, Robbins JS, Phillips ST (2014) A prototype point-of-use assay for measuring heavy metal contamination in water using time as a quantitative readout. Chem Commun 50:5352–5354
Li H, Steckl AJ (2018) Paper microfluidics for point-of-care blood-based analysis and diagnostics. Anal Chem 91:352–371
Li X, Tian J, Nguyen T, Shen W (2008) Paper-based microfluidic devices by plasma treatment. Anal Chem 80:9131–9134
Li M, Tian J, Al-Tamimi M, Shen W (2012) Paper-based blood ty** device that reports patient’s blood type “in Writing.” Angew Chem Int Ed 51:5497–5501
Li H, Han D, Pauletti GM, Steckl AJ (2014) Blood coagulation screening using a paper-based microfluidic lateral flow device. Lab chip 14:4035–4041. https://doi.org/10.1039/c4lc00716f
Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH (2013) Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal Chem 85:10463–10470. https://doi.org/10.1021/ac402459h
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed 46:1318–1320
Martinez AW, Phillips ST, Carrilho E, Thomas SW III, Sindi H, Whitesides GM (2008) Simple telemedicine for develo** regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 80:3699–3707
Morbioli GG, Mazzu-Nascimento T, Stockton AM, Carrilho E (2017) Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs)—a review. Anal Chim Acta 970:1–22
Mukhopadhyay SM, Joshi P, Datta S, Macdaniel J (2002) Plasma assisted surface coating of porous solids. Appl Surf Sci 201:219–226. https://doi.org/10.1016/S0169-4332(02)00939-X
Nie J, Liang Y, Zhang Y, Le S, Li D, Zhang S (2013) One-step patterning of hollow microstructures in paper by laser cutting to create microfluidic analytical devices. Analyst 138:671–676
Noiphung J, Songjaroen T, Dungchai W, Henry CS, Chailapakul O, Laiwattanapaisal W (2013) Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal Chim Acta 788:39–45
Olkkonen J, Lehtinen K, Erho T (2010) Flexographically printed fluidic structures in paper. Anal Chem 82:10246–10250
OuYang L, Wang C, Du F, Zheng T, Liang H (2014) Electrochromatographic separations of multi-component metal complexes on a microfluidic paper-based device with a simplified photolithography RSC. Adv 4:1093–1101
Park C, Kim H-R, Kim S-K, Jeong I-K, Pyun J-C, Park S (2019) Three-dimensional paper-based microfluidic analytical devices integrated with a plasma separation membrane for the detection of biomarkers in whole blood ACS App. Mater Interfaces 11:36428–36434. https://doi.org/10.1021/acsami.9b13644
Pollock NR et al (2012) A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med 4:152ra129
Punpattanakul K, Kraduangdej S, Jiranusornkul N, Chiaranairungroj M, Pimpin A, Palaga T, Srituravanich W (2018) A novel patterning method for three-dimensional paper-based devices by using inkjet-printed water mask. Cellulose 25:2659–2665. https://doi.org/10.1007/s10570-018-1712-8
Raj N, Breedveld V, Hess DW (2019) Fabrication of fully enclosed paper microfluidic devices using plasma deposition and etching. Lab Chip 19:3337–3343
Raj N, Breedveld V, Hess DW (2020) Flow control in fully enclosed microfluidics paper based analytical devices using plasma processes. Sens Actuat B Chem 320:128606
Reboud J et al (2019) Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc Natl Acad Sci 116:4834–4842
Renault C, Li X, Fosdick SE, Crooks RM (2013) Hollow-channel paper analytical devices. Anal Chem 85:7976–7979
Renault C, Koehne J, Ricco AJ, Crooks RM (2014) Three-dimensional wax patterning of paper fluidic devices. Langmuir 30:7030–7036
Schilling KM, Lepore AL, Kurian JA, Martinez AW (2012) Fully enclosed microfluidic paper-based analytical devices. Anal Chem 84:1579–1585
Schindelin J et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Songjaroen T, Dungchai W, Chailapakul O, Henry CS, Laiwattanapaisal W (2012) Blood separation on microfluidic paper-based analytical devices. Lab Chip 12:3392–3398
Talbot EL, Yang L, Berson A, Bain CD (2014) Control of the particle distribution in inkjet printing through an evaporation-driven sol–gel transition. ACS Appl Mater Interfaces 6:9572–9583
Tan W, Zhang L, Doery JCG, Shen W (2020) Three-dimensional microfluidic tape-paper-based sensing device for blood total bilirubin measurement in jaundiced neonates. Lab chip 20:394–404. https://doi.org/10.1039/C9LC00939F
Tang RH et al (2017) Advances in paper-based sample pretreatment for point-of-care testing. Crit Rev Biotechnol 37:411–428
Tian T, Bi Y, Xu X, Zhu Z, Yang C (2018) Integrated paper-based microfluidic devices for point-of-care testing. Anal Methods 10:3567–3581. https://doi.org/10.1039/C8AY00864G
Van Zyl L (1967) Normal values for serum protein fractions in sheep as obtained by electrophoresis on cellulose acetate strips J. Vet Res 34:633–646
Vella SJ et al (2012) Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal Chem 84:2883–2891
Yang XX, Forouzan O, Brown TP, Shevkoplyas SS (2012) Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab chip 12:274–280. https://doi.org/10.1039/c1lc20803a
Zhang H, Smith E, Zhang W, Zhou A (2019) Inkjet printed microfluidic paper-based analytical device (μPAD) for glucose colorimetric detection in artificial urine. Biomed Microdev 21:1–10
Zhang H, Chen Z, Dai J, Zhang W, Jiang Y, Zhou A (2021) A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem J 162:105814
Zhou F, Noor MO, Krull UJ (2014) Luminescence resonance energy transfer-based nucleic acid hybridization assay on cellulose paper with upconverting phosphor as donors. Anal Chem 86:2719–2726
Zhu W-J, Feng D-Q, Chen M, Chen Z-D, Zhu R, Fang H-L, Wang W (2014) Bienzyme colorimetric detection of glucose with self-calibration based on tree-shaped paper strip. Sens Actuat B Chem 190:414–418
Acknowledgments
The authors thank Dr. Ashwini Sinha at Linde for donating the pentafluoroethane precursor. The authors also thank Jianshan Liao (Georgia Tech) for preparing the CNC suspensions.
Funding
There is no funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raj, N., Breedveld, V. & Hess, D.W. Semi-enclosed microfluidic device on glass-fiber membrane with enhanced signal quality for colorimetric analyte detection in whole blood. Microfluid Nanofluid 25, 47 (2021). https://doi.org/10.1007/s10404-021-02447-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-021-02447-6