Abstract
Purpose
To evaluate vision-related quality of life (QoL) in wet age-related macular degeneration (wAMD) patients receiving intravitreal aflibercept (IVT-AFL).
Study design
Prospective, observational Japanese postmarketing surveillance study.
Methods
All decisions were made by the treating physician. QoL was assessed using the 25-item National Eye Institute-Visual Functioning Questionnaire (NEI-VFQ-25) composite score administered at baseline, 6 months, and 12 months (primary assessment). Secondary assessments included NEI-VFQ-25 subscale scores, resource use, and best-corrected visual acuity (BCVA; logarithm of the minimum angle of resolution [logMAR]).
Results
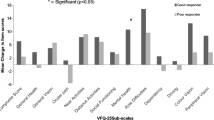
In total, 576 patients (baseline), 555 patients (6 months), and 446 patients (12 months) were included. The mean (SD) number of IVT-AFL injections was 3.5 (1.2) at 6 months and 4.6 (2.2) at 12 months. The mean (SD) improvement from baseline in the NEI-VFQ-25 composite score was 3.1 (11.1) at 6 months and 2.7 (12.3) at 12 months (P < .0001). For the NEI-VFQ-25 subscale scores, the mean change was ≥ 4 (minimally important difference) for general vision, near vision, and mental health at 6 months, and for general vision and mental health at 12 months (all P < .0001). A significant improvement from baseline was found in mean BCVA (logMAR) at 6 months (-0.1) and 12 months (-0.1) (P < .0001). The mean change from baseline in the NEI-VFQ-25 scores was greatest in patients with improved BCVA (gain of ≤ -0.3 logMAR units or ≥ 15 letters) after treatment.
Conclusion
IVT-AFL was associated with significant improvements in QoL and visual acuity in Japanese patients with wAMD in a real-world setting.





Similar content being viewed by others
References
Tamura H, Goto R, Akune Y, Hiratsuka Y, Hiragi S, Yamada M. The clinical effectiveness and cost-effectiveness of screening for age-related macular degeneration in Japan: a Markov modeling study. PLoS One. 2015;10:e0133628.
Nakae K, Masuda K, Senoo T, Sawa M, Kanai J, Ishibashi T. Aging society and eye disease: a recent epidemiological study on underlying diseases responsible for visual impairment. Geriatr Med. 2006;44:1221–4 (in Japanese).
Wako R, Yasukawa T, Kato A, Omori T, Ishida S, Ishibashi T, et al. Causes and prevalence of visual impairment in Japan. Nippon Ganka Gakkai Zasshi. 2014;118:495–501 (in Japanese).
Yasuda M, Kiyohara Y, Hata Y, Arakawa S, Yonemoto K, Doi Y, et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population: the Hisayama study. Ophthalmology. 2009;116:2135–40.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16.
Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–2.
Yuzawa M, Fujita K, Tanaka E, Wang EC. Assessing quality of life in the treatment of patients with age-related macular degeneration: clinical research findings and recommendations for clinical practice. Clin Ophthalmol. 2013;7:1325–32.
Roberts CB, Hiratsuka Y, Yamada M, Pezzullo ML, Yates K, Takano S, et al. Economic cost of visual impairment in Japan. Arch Ophthalmol. 2010;128:766–71.
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I, Wong TY, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107–39.
** J, Yuan F, Shen MQ, Feng YF, He QL. Vascular endothelial growth factor regulates primate choroid-retinal endothelial cell proliferation and tube formation through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem. 2013;381:267–72.
Takahashi K, Ogura Y, Ishibashi T, Shiraga F, Yuzawa M. Treatment guidelines for age-related macular degeneration. Nippon Ganka Gakkai Zasshi. 2012;116:1150–5 (in Japanese).
Yuzawa M, Fujita K, Wittrup-Jensen KU, Norenberg C, Zeitz O, Adachi K, et al. Improvement in vision-related function with intravitreal aflibercept: data from phase 3 studies in wet age-related macular degeneration. Ophthalmology. 2015;122:571–8.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Ogura Y, Terasaki H, Gomi F, Yuzawa M, Iida T, Honda M, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. Br J Ophthalmol. 2015;99:92–7.
Ministry of Health, Labour and Welfare. Ministerial ordinance on good post-marketing study practice for drugs. MHLW Ordinance No. 171 of 2004.
Eylea (package insert [in Japanese]). Tokyo, Japan: Pharmaceuticals and Medical Devices Agency; 2016.
Suzukamo Y, Oshika T, Yuzawa M, Tokuda Y, Tomidokoro A, Oki K, et al. Psychometric properties of the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25), Japanese version. Health Qual Life Outcomes. 2005;3:65.
Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30:1046–50.
Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009;50:3629–35.
Rakic JM, Leys A, Brie H, Denhaerynck K, Pacheco C, Vancayzeele S, et al. Real-world variability in ranibizumab treatment and associated clinical, quality of life, and safety outcomes over 24 months in patients with neovascular age-related macular degeneration: the HELIOS study. Clin Ophthalmol. 2013;7:1849–58.
Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology. 2010;117(747–56):e4.
Acknowledgments
The statistical analyses were performed by CMIC and were funded by Bayer Yakuhin. Medical writing assistance was provided by PAREXEL and funded by Bayer Yakuhin. Study group investigators: The Participating Investigators were Tsukasa Hanemoto, Kazuaki Nishijima, Hisashi Matsubara, Harumi Wakiyama, Sachio Kawashima, Hiroaki Sato, Shigeki Tagawa, Toyohisa Yoshizawa, Toshiaki Kurakazu, Kenji Inoue, Takatomo Miyake, Koichi Ota, Kunihiko Shiraki, Yoshihide Nakai, Yasuo Kurimoto, Shunji Kusaka, Shigeru Hoshiai, Akihiko Ohira, Masahiro Morimoto, Hitoshi Tabuchi, Kaori Sato, Yasushi Inoue, Sakura Sato, Naomichi Katai, Akira Obana, Junkichi Kabayama, Hidetaka Yamaji, Shiro Ozaki, Jun Sueda, Hidetoshi Hanasaki, Tadayuki Nishide, Hirofumi Yonezawa, Koji Kawamoto, Hidenori Kamimoto, Atsushi Hayashi, Miki Honda, Masahiro Miura, Ryo Obata, Isao Saito, Hideyasu Oh, Takatoshi Maeno, Kazuki Ishibashi, Yoko Fukushima, Taiji Sakamoto, Akiteru Kawahara, Tetsuo Ogino, Jun Arai, Hiroyuki Sato, Yosuke Ida, Satoshi Nakamura, Hirotaka Yokouchi, Misa Suzuki, Kazuyuki Hara, Yasukazu Hino, Ryo Watanabe, Keiichi Mitarai, Kazuhisa Miyamoto, Masayuki Akimoto, Kiyoshi Ishii, Hitoshi Takagi, Naotaka Kanda, Takeo Fukuchi, Masahiro Kaneda, Hiroki Tsu**aka, Hisaharu Suzuki, Yasuhiro Tanifuji, Akinori Uemura, Tsunehiko Ikeda, Hiroko Imaizumi, Kenji Yoshida, Susumu Ishida, Tomoki Sakuraba, and Yukihiro Horie. All Participating Investigators provided and cared for the study patients and collected data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
F. Gomi, Honorarium (Bayer, Alcon, Novartis, Santen, Senju), Speaker fee (Alcon, Bayer, Santen, Senju), Advisory board fee (Alcon, Bayer, Senju), Grant (Alcon, Bayer, Johnson and Johnson, Nidek, Novartis, Pfizer, Santen, Senju), H. Migita, Employee (Bayer); T. Sakaguchi, Employee (Bayer); H. Okada, Consultant fee (Bayer); T. Sugawara, Consultant fee (Bayer); Y. Hikichi, Employee (Bayer).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Yusuke Hikichi
The members of The Participating Investigators are listed in “Acknowledgements” section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Gomi, F., Migita, H., Sakaguchi, T. et al. Vision-related quality of life in Japanese patients with wet age-related macular degeneration treated with intravitreal aflibercept in a real-world setting. Jpn J Ophthalmol 63, 437–447 (2019). https://doi.org/10.1007/s10384-019-00687-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-019-00687-2