Abstract
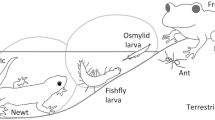
The chemical defense of insects is effective for avoiding predation, but may carry a cost in terms of life history traits. If chemical defenses require the resources and/or nutrients necessary for the larva or post-larval stages to survive, grow, and reproduce, there will be a trade-off between chemical defense and other traits, particularly in habitats where larvae are subjected to frequent predator attacks. The larvae of Osmylus hyalinatus McLachlan (Neuroptera: Osmylidae) are semiaquatic, inhabiting the edges of small streams and ponds, where they encounter multiple predators both on land and in water. Larvae of this species spray a hyaline liquid from an anal opening when disturbed. The liquid is stored in the posterior half of the hindgut. Daily stimulation of larvae to exhaust the stored liquid, thereby simulating repeated predator attacks, resulted in smaller adult body size at emergence than the control, but had little effect on the larval/pupal period, cocoon production (for predator avoidance of prepupae and pupae), reproductive potential, or chemical defense of adults in which prothoracic glands release a substance that smells unpleasant to predators. The lack of such effects is explained in part by adults gaining more resources through feeding than the larval stages, as well as nuptial gifts from males to females. The spraying liquids and silk used to spin cocoons are both discharged from an anal opening; therefore, a trade-off between these two materials is plausible and should be examined in the future.




Similar content being viewed by others
Data availability
The authors make all the data available to readers upon request.
References
Aldrich JR, Zhang QH (2016) Chemical ecology of neuroptera. Ann Rev Entomol 61:197–218
Aldrich JR, Le TC, Zhang QH, Torres J, Winterton SL, Han B, Miller GL, Chauhan KR (2009) Prothoracic gland semiochemicals of green lacewings. J Chemical Ecol 35:1181–1187
Bayoumy MH, Osawa N, Hatt S (2020) Fitness costs of reflex bleeding in the ladybird beetle Harmonia axyridis: the role of parental effects. Insect Sci 27:1346–1359
Beran F, Petschenka G (2022) Sequestration of plant defense compounds by insects: from mechanisms to insect–plant coevolution. Ann Rev Entomol 67:163–180
Blum MS, Wallace JB, Fales HM (1973) Skatole and tridecene: identification and possible role in a chrysopid secretion. Insect Biochem 3:353–357
Bowers MD (1992) The evolution of unpalatability and the cost of chemical defense in insects. In: Roitberg BD, Isman MB (eds) Insect chemical ecology. An evolutionary approach. Chapman & Hall, New York, pp 216–244
Camara MD (1997) Physiological mechanisms underlying the costs of chemical defence in Junonia coenia Hübner (Nymphalidae): a gravimetric and quantitative genetic analysis. Evol Ecol 11:451–469
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85:139–169
Dettner K (2015) Toxins, defensive compounds and drugs from insects. In: Hoffmann KH (ed) Insect molecular biology and ecology. CRC, Boca Raton, FL, pp 39–93
Dettner K (2019) Defenses of water insects. In: Del-Claro K, Guillermo R (eds) Aquatic insects. Springer, Cham, pp 191–262
Devetak D, Klokočovnik V (2016) The feeding biology of adult lacewings (Neuroptera): a review. Trends in Entomol 12:30–42
Eisner T, Eisner M, Siegler M (2005) Secret weapons. Defenses of insects, spiders, scorpions, and other many-legged creatures. Harvard University Press, Cambridge
Grill CP, Moore AJ (1998) Effects of a larval antipredator response and larval diet on adult phenotype in an aposematic ladybird beetle. Oecologia 114:274–282
Güsten R (1996) A review of epidermal glands in the order Neuroptera (Insecta). In: Canard M, Aspöck H, Mansell MW (eds) Pure and applied research in neuropterology. Proceedings of the fifth international symposium on neuropterology, Toulouse, France, pp 129–146
Güsten R, Dettner K (1991) The prothoracic gland of the Chrysopidae (Neuropteroidea: Planipennia). In: Zombori L, Peregovits L (eds) Proceedings of the 4th European congress of entomology and the XIII internationale Symposium für die entomofaunistik Mitteleuropas, Hungrian Natural History Museum, Budapest, pp 60–65
Hartmann T (2004) Plant-derived secondary metabolites as defensive chemicals in herbivorous insects: a case study in chemical ecology. Planta 219:1–4
Hayashi F (1992) Large spermatophore production and consumption in dobsonflies Protohermes (Megaloptera, Corydalidae). Kontyû 60:59–66
Hayashi F (1993) Male mating costs in two insect species (Protohermes, Megaloptera) that produce large spermatophores. Anim Behav 45:343–349
Hayashi F (1998) Multiple mating and lifetime reproductive output in female dobsonflies that receive nuptial gifts. Ecol Res 13:283–289
Hayashi F (2018) Neuroptera. In: Kawai T, Tanida K (eds) Aquatic insects of Japan: manual with keys and illustrations, 2nd edn. Tokai University Press, Kanagawa, pp 437–442 ((In Japanese))
Higginson AD, Delf J, Ruxton GD, Speed MP (2011) Growth and reproductive costs of larval defence in the aposematic lepidopteran Pieris brassicae. J Anim Ecol 80:384–392
Iwanami T, Yu P, Hayashi F (2021) Defensive spray by a semiaquatic osmylid larva (Insecta: Neuroptera) for both aquatic and terrestrial predators. J Ethol 39:369–377
Jandausch K, Beutel RG, Bellstedt R (2019) The larval morphology of the spongefly Sisyra nigra (Retzius, 1783) (Neuroptera: Sisyridae). J Morphol 280:1742–1758
Kearsley MJ, Whitham TG (1992) Guns and butter: a no cost defense against predation for Chrysomela confluens. Oecologia 92:556–562
Kingsolver JG, Huey RB (2008) Size, temperature, and fitness: three rules. Evol Ecol Res 10:251–268
Knapp M, Řeřicha M, Židlická D (2020) Physiological costs of chemical defence: repeated reflex bleeding weakens the immune system and postpones reproduction in a ladybird beetle. Sci Rep 10:1–7
LaMunyon C (1988) Hindgut changes preceding pupation and related cocoon structure in Chrysoperla comanche Banks (Neuroptera, Chrysopidae). Psyche 95:203–209
LaMunyon CW, Adams PA (1987) Use and effect of an anal defensive secretion in larval Chrysopidae (Neuroptera). Ann Entomol Soc Am 80:804–808
Lee BW, Ugine TA, Losey JE (2018) An assessment of the physiological costs of autogenous defenses in native and introduced lady beetles. Environ Entomol 47:1030–1038
Lewis SM, Vahed K, Koene JM, Engqvist L, Bussiere LF, Perry JC, Gwynne D, Lehmann GU (2014) Emerging issues in the evolution of animal nuptial gifts. Biol Lett 10:20140336
Lindstedt C, Miettinen A, Freitak D, Ketola T, López-Sepulcre A, Mäntylä E, Pakkanen H (2018) Ecological conditions alter cooperative behaviour and its costs in a chemically defended sawfly. Proceed Roy Soc B 285:20180466
Lindstedt C, Murphy L, Mappes J (2019) Antipredator strategies of pupae: how to avoid predation in an immobile life stage? Philos Trans R Soc B 374:20190069
Lindstedt C, Suisto K, Burdfield-Steel E, Winters AE, Mappes J (2020) Defense against predators incurs high reproductive costs for the aposematic moth Arctia plantaginis. Behav Ecol 31:844–850
Liu X, Hayashi F, Lavine LC, Yang D (2015) Is diversification in male reproductive traits driven by evolutionary trade-offs between weapons and nuptial gifts? Proceed Roy Soc B 282:20150247
Martins CC, Ardila-Camacho A (2018) Order neuroptera. In: Hamada N, Thorp JH, Rogers DC (eds) Keys to neotropical hexapoda, 4th edition: thorp and Covich’s freshwater invertebrates, vol 3. Academic Press, Cambridge, pp 229–236
Pacheco CA, Alevi KCC, Ravazi A, Oliveira MTVDA (2014) Malpighian tubule, an essential organ for insects. Entomol Ornithol Herpetol 3:122
Pacheco P, Borges I, Branco B, Lucas E, Soares AO (2021) Costs and benefits of wax production in the larvae of the ladybeetle Scymnus nubilus. Insects 12:458
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rowell-Rahier M, Pasteels JM (1986) Economics of chemical defense in Chrysomelinae. J Chem Ecol 12:1189–1203
Sato S, Kushibuchi K, Yasuda H (2009) Effect of reflex bleeding of a predatory ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), as a means of avoiding intraguild predation and its cost. Appl Entomol Zool 44:203–206
Smilanich AM, Dyer LA, Chambers JQ, Bowers MD (2009) Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol Lett 12:612–621
Spiegler PE (1962) The origin and nature of the adhesive substance in larvae of the genus Chrysopa (Neuroptera: Chrysopidae). Ann Entomol Soc Am 55:69–77
Sugiura S (2020) Predators as drivers of insect defenses. Entomol Sci 23:316–337
Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ (2010) Insect silk: one name, many materials. Ann Rev Entomol 55:171–188
Walker AA, Robinson SD, Yeates DK, ** J, Baumann K, Dobson J, Fry BG, King GF (2018) Entomo-venomics: the evolution, biology and biochemistry of insect venoms. Toxicon 154:15–27
Zimmermann D, Randolf S, Aspöck U (2019) From chewing to sucking via phylogeny–from sucking to chewing via ontogeny: mouthparts of Neuroptera. In: Krenn HW (ed) Insect mouthparts, zoological monographs 5. Springer, Cham, pp 361–385
Zvereva EL, Kozlov MV (2016) The costs and effectiveness of chemical defenses in herbivorous insects: a meta-analysis. Ecol Monogr 86:107–124
Zvereva EL, Zverev V, Kruglova OY, Kozlov MV (2017) Strategies of chemical anti-predator defences in leaf beetles: is sequestration of plant toxins less costly than de novo synthesis? Oecologia 183:93–106
Acknowledgements
We thank Yasukazu Okada for offering the worker ants of Formica japonica as the model predator from his cultured colonies and the two anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the completion of this study; particularly, PY and FH designed the study, TI and KS collected materials, and PY, TI, and FH performed rearing experiments, HY, MT, and FH performed the lizard predation experiment, and PY and FH analyzed all data and wrote the manuscript which was finally checked by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines (A3-3 in Tokyo Metropolitan University) for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yu, P., Iwanami, T., Yazaki, H. et al. Cost of defensive spraying by larval Osmylus hyalinatus (Neuroptera: Osmylidae) for post-larval development. J Ethol 41, 129–139 (2023). https://doi.org/10.1007/s10164-023-00779-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-023-00779-0