Abstract
Background
Enfortumab vedotin is a novel antibody–drug conjugate used as a third-line therapy for the treatment of urothelial cancer. We aimed to elucidate the effect of enfortumab vedotin-related peripheral neuropathy on its efficacy and whether enfortumab vedotin-induced early electrophysiological changes could be associated with peripheral neuropathy onset.
Methods
Our prospective multicenter cohort study enrolled 34 patients with prior platinum-containing chemotherapy and programmed cell death protein 1/ligand 1 inhibitor-resistant advanced urothelial carcinoma and received enfortumab vedotin. The best overall response, progression-free survival, overall survival, and safety were assessed. Nerve conduction studies were also performed in 11 patients.
Results
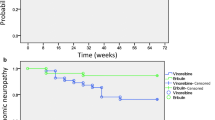
The confirmed overall response rate and disease control rate were 52.9% and 73.5%, respectively. The median overall progression-free survival and overall survival were 6.9 and 13.5 months, respectively, during a median follow-up of 8.6 months. The patients with disease control had significantly longer treatment continuation and overall survival than did those with uncontrolled disease. Peripheral neuropathy occurred in 12.5% of the patients. The overall response and disease control rates were 83.3% and 100%, respectively: higher than those in patients without peripheral neuropathy (p = 0.028 and p = 0.029, respectively). Nerve conduction studies indicated that enfortumab vedotin reduced nerve conduction velocity more markedly in sensory nerves than in motor nerves and the lower limbs than in the upper limbs, with the sural nerve being the most affected in the patients who developed peripheral neuropathy (p = 0.011).
Conclusion
Our results indicated the importance of focusing on enfortumab vedotin-induced neuropathy of the sural nerve to maximize efficacy and improve safety.



Similar content being viewed by others
Data availability
The data supporting the findings in this study are available within the paper, in the corresponding Online Resource, or upon reasonable request from the corresponding author.
References
Rosenberg J, Sridhar SS, Zhang J et al (2020) EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 38:1041–1049
Powles T, Rosenberg JE, Sonpavde GP et al (2021) Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384:1125–1135
Rosenberg JE, Powles T, Sonpavde GP et al (2023) EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin vs chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann Oncol 34:1047–1054
Loprinzi CL, Lacchetti C, Bleeker J et al (2020) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 38:3325–3348
Fateh HR, Metdani SP (2021) Role of interdigital sensory nerve conduction study as a noninvasive approach for early diagnosis of diabetic peripheral neuropathy. J Diabetes Metab Disord 20:71–75
Vlachou E, Matoso A, McConkey D et al (2023) Enfortumab vedotin-related cutaneous toxicity and radiographic response in patients with urothelial cancer: a single-center experience and review of the literature. Eur Urol Open Sci 49:100–103
Powles T, Bellmunt J, Comperat E et al (2022) Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33:244–258
Bladder Cancer, version 3 (2023). National comprehensive cancer network. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed 12 October 2023
von der Maase H, Hansen SW, Roberts JT et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
Sternberg CN, de Mulder P, Schornagel JH et al (2006) Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42:50–54
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33
Bellmunt J, de Wit R, Vaughn DJ et al (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015–1026
Fradet Y, Bellmunt J, Vaughn DJ et al (2019) Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 30:970–976
Powles T, Park SH, Voog E et al (2020) Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218–1230
Matsubara N, Yonese J, Kojima T et al (2023) Japanese subgroup analysis of EV-301: an open-label, randomized phase 3 study to evaluate enfortumab vedotin versus chemotherapy in subjects with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Med 12:2761–2771
Best RL, LaPointe NE, Azarenko O et al (2021) Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol Appl Pharmacol 421:115534
Alberti P, Rossi E, Argyriou AA et al (2018) Risk stratification of oxaliplatin induced peripheral neurotoxicity applying electrophysiological testing of dorsal sural nerve. Support Care Cancer 26:3143–3151
Velasco R, Bruna J, Briani C et al (2014) Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 85:392–398
Chen X, Stubblefield MD, Custodio CM et al (2013) Electrophysiological features of taxane-induced polyneuropathy in patients with breast cancer. J Clin Neurophysiol 30:199–203
Acknowledgements
The authors thank the patients and their families who were involved in this post-marketing survey and the physicians who cooperated with the survey and data collection.
Funding
This study did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
All the authors had access to the complete data, significantly contributed to development of the manuscript, and had final responsibility for the decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
The objective response and outcome of EV treatment in the patients with and without peripheral neuropathy. Supplementary file1 (JPG 103 KB)
Appendix
Appendix
Study institutes: Kagawa University, Takamatsu Red Cross Hospital, Kagawa Prefectural Central Hospital, Tokushima University Graduate School of Biomedical Sciences, Sakaide City Hospital, Mitoyo General Hospital, Shodoshima Central Hospital, Kansai Medical University, Kyoto University Graduate School of Medicine and KKR Takamatsu Hospital.
About this article
Cite this article
Taoka, R., Kamada, M., Izumi, K. et al. Peripheral neuropathy and nerve electrophysiological changes with enfortumab vedotin in patients with advanced urothelial carcinoma: a prospective multicenter cohort study. Int J Clin Oncol 29, 602–611 (2024). https://doi.org/10.1007/s10147-024-02481-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02481-8