Abstract
Background
Cabazitaxel has played an important role in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC); however, several types of sequential therapy against mCRPC have been performed in routine clinical practice. The objective of this study was to investigate the impact of third-line treatment on prognostic outcomes of mCRPC patients.
Methods
This study retrospectively analyzed the clinical outcomes of 166 patients who received 3 agents following the diagnosis of mCRPC, consisting of 81 sequentially treated with either abiraterone or enzalutamide and then docetaxel, followed by third-line cabazitaxel (group A) and 85 treated with 3 agents, including abiraterone, enzalutamide, and docetaxel (group B).
Results
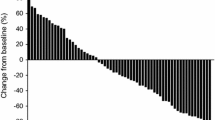
There were no significant differences in major characteristics at the introduction of the third-line agent between these 2 groups. The proportion of patients with prostate-specific antigen (PSA) reduction > 50% by cabazitaxel in group A was significantly greater than that by either third-line agent in group B. Both PSA progression-free survival (PFS) and overall survival (OS) following third-line therapy in group A were significantly longer than those in group B. Furthermore, OS after the diagnosis of mCRPC in group A was significantly longer than that in group B. Multivariate analysis identified independent predictors of favorable prognostic outcomes after third-line therapy as follows: high-performance status (PS), low PSA level and third-line cabazitaxel for PSA PFS, and high PS, low lactate dehydrogenase level and third-line cabazitaxel for OS.
Conclusions
The introduction of cabazitaxel as a third-line agent could markedly improve the prognostic outcomes of mCRPC patients.


Similar content being viewed by others
References
Nuhn P, De Bono JS, Fizazi K et al (2019) Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol 75:88–99
Ingrosso G, Detti B, Scartoni D et al (2018) Current therapeutic options in metastatic castration-resistant prostate cancer. Semin Oncol 45:303–315
Abidi A (2013) Cabazitaxel: a novel taxane for metastatic castration-resistant prostate cancer-current implications and future prospects. J Pharmacol Pharmacother 4:230–237
de Bono JS, Oudard S, Ozguroglu M et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
Miyake H, Hara T, Tamura K et al (2017) Comparative assessment of efficacies between 2 alternative therapeutic sequences with novel androgen receptor-axis-targeted agents in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 15:e591-597
Mori K, Miura N, Mostafaei H et al (2020) Sequential therapy of abiraterone and enzalutamide in castration-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 23:539–548
Fizazi K, Scher HI, Molina A et al (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13:983–992
Scher HI, Fizazi K, Saad F et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187–1197
de Wit R, de Bono J, Sternberg CN et al (2019) Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 381:2506–2518
Scher HI, Halabi S, Tannock I et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148–1159
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Miyake H, Sakai I, Harada K et al (2012) Significance of docetaxel-based chemotherapy as treatment for metastatic castration-resistant prostate cancer in Japanese men over 75 years old. Int Urol Nephrol 44:1697–1703
Soloway MS, Hardeman SW, Hickey D et al (1988) Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 61:195–202
Carles J, Pichler A, Korunkova H et al (2019) An observational, multicentre study of cabazitaxel in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (CAPRISTANA). BJU Int 123:456–464
Miyake H, Sugiyama T, Aki R et al (2017) No significant impact of prior treatment profile with docetaxel on the efficacy of cabazitaxel in Japanese patients with metastatic castration-resistant prostate cancer. Med Oncol 34:141
Ito T, Kanao K, Takahara K et al (2019) Optimal timing of cabazitaxel introduction for Japanese patients with metastatic castration-resistant prostate cancer. Anticancer Res 39:3089–3094
Joly F, Oudard S, Fizazi K et al (2020) Quality of life and pain during treatment of metastatic castration-resistant prostate cancer with cabazitaxel In routine clinical practice. Clin Genitourin Cancer 18:e510-516
Malik Z, Heidenreich A, Bracarda S et al (2019) Real-world experience with cabazitaxel in patients with metastatic castration-resistant prostate cancer: a final, pooled analysis of the compassionate use programme and early access programme. Oncotarget 10:4161–4168
Fitzpatrick JM, de Wit R (2014) Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol 65:1198–1204
Antonarakis ES, Lu C, Wang H et al (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371:1028–1038
Conteduca V, Castro E, Wetterskog D et al (2019) Plasma AR status and cabazitaxel in heavily treated metastatic castration resistant prostate cancer. Eur J Cancer 116:158–168
Shiota M, Nakamura M, Yokomizo A et al (2020) Prognostic significance of lactate dehydrogenase in cabazitaxel chemotherapy for castration-resistant prostate cancer: a multi-institutional study. Anticancer Drugs 31:298–303
Rouyer M, Oudard S, Joly F et al (2019) Overall and progression-free survival with cabazitaxel in metastatic castration-resistant prostate cancer in routine clinical practice: the FUJI cohort. Br J Cancer 121:1001–1008
Terada N, Kamoto T, Tsukino H et al (2019) The efficacy and toxicity of cabazitaxel for treatment of docetaxel-resistant prostate cancer correlating with the initial doses in Japanese patients. BMC Cancer 19:156
Fizazi K, Kramer G, Eymard JC et al (2020) Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol 21:1513–1525
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Miyake, R. Sato, K. Watanabe, Y. Matsushita, H. Watanabe, D. Motoyama, T. Ito, T. Sugiyama and A. Otsuka declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study given the retrospective nature of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Miyake, H., Sato, R., Watanabe, K. et al. Prognostic significance of third-line treatment for patients with metastatic castration-resistant prostate cancer: comparative assessments between cabazitaxel and other agents. Int J Clin Oncol 26, 1745–1751 (2021). https://doi.org/10.1007/s10147-021-01956-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01956-2