Abstract
Background
We aimed to evaluate the factors predicting efficacy and adverse effects of enzalutamide in patients with castration-resistant prostate cancer.
Methods
We retrospectively evaluated data on 345 patients who had received enzalutamide for castration-resistant prostate cancer in 20 hospitals (Kyoto University Hospital and other satellite hospitals). Cox proportional hazards regression analysis was performed to identify factors predicting prostate-specific antigen (PSA) progression after enzalutamide treatment and logistic regression analysis for those associated with development of adverse effects.
Results
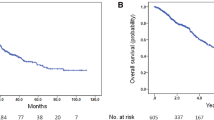
PSA titers decreased by >50 % in 197 patients (57 %). The median PSA progression free survival was 163 days. Gleason score >8 (HR 2.078, 95 % CI 1.37–3.153, P = 0.00058), performance status ≥1 (HR 2.292, 95 % CI 1.463–3.592, P = 0.000296), presence of bone metastasis (HR 1.774, 95 % CI 1.019–3.090, P = 0.0429), visceral metastasis (HR 2.127, 95 % CI 1.215–3.722, P = 0.00823), previous steroid treatment (HR 1.780, 95 % CI 1.207–2.626, P = 0.00361) and docetaxel treatment (HR 1.602, 95 % CI 1.051–2.442, P = 0.0284) significantly predicted the efficacy of enzalutamide. Adverse effects, including fatigue or appetite loss, occurred in 169 patients (49 %), 48 (18 %) of whom stopped enzalutamide. Age >75 years (HR 1.980, 95 % CI 1.270–3.09, P = 0.00246) and lower enzalutamide dose (HR 0.437, 95 % CI 0.255–1.270, P = 0.00249) were significantly associated with development of adverse effects.
Conclusions
Enzalutamide treatment is effective in patients with castration-resistant prostate cancer with low Gleason scores, good performance status, without bone or visceral metastasis and no prior steroid or docetaxel treatment. Lower doses of enzalutamide decrease the incidence of adverse effects, especially in older patients.


Similar content being viewed by others
References
Siegel R, Ma J, Zou Z et al (2014) Cancer statistics. CA Cancer J Clin 64(1):9–29. doi:10.3322/caac.21208
Ito K (2014) Prostate cancer in Asian men. Nat Rev Urol 11(4):197–212. doi:10.1038/nrurol.2014.42
Harris WP, Mostaghel EA, Nelson PS et al (2009) Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 6(2):76–85. doi:10.1038/ncpuro1296
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15):1502–1512. doi:10.1056/NEJMoa040720
Armstrong AJ, Eisenberger MA, Halabi S et al (2012) Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol 61(3):549–559. doi:10.1016/j.eururo.2011.11.009
Scher HI, Fizazi K, Saad F et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367(13):1187–1197. doi:10.1056/NEJMoa1207506
Beer TM, Tombal B (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371(18):1755–1756. doi:10.1056/NEJMc1410239
Antonarakis ES, Lu C, Wang H et al (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371(11):1028–1038. doi:10.1056/NEJMoa1315815
Goodman OB Jr, Flaig TW, Molina A et al (2014) Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 17(1):34–39. doi:10.1038/pcan.2013.41
Ryan CJ, Molina A, Li J et al (2013) Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized phase III trial. J Clin Oncol 31(22):2791–2798. doi:10.1200/JCO.2012.45.4595
Arora VK, Schenkein E, Murali R et al (2013) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155(6):1309–1322. doi:10.1016/j.cell.2013.11.012
Narayanan S, Srinivas S, Feldman D (2015) Androgen-glucocorticoid interactions in the era of novel prostate cancer therapy. Nat Rev Urol. doi:10.1038/nrurol.2015.254
Acknowledgments
The authors wish to acknowledge the assistance of the following in this study: Takehiko Segawa, Toru Yoshida, Kyoto City Hospital; Hiroshi Okuno, Kyoto Medical Center; Mitsuo Nonomura, Norio Kawase, Kyoto Katsura Hospital; ** Yamada, Toru Kanno, I**kai Takeda Hospital; Tomoyuki Oida, Kosekai Takeda Hospital; Toshiya Akao, Noboru Shibasaki, Otowa Hospital; Keiji Ogura, Toru Isitoya, Otsu Red Cross Hospital; Yasumasa Shichiri, Akihiro Hamada, Otsu Municipal Hospital; Hiroyuki Onishi, Koji Nishizawa, Shiga Medical Center for Adults; Kazuo Nishimura, Kazutoshi Okubo, Osaka Red Cross Hospital; Jun Takenawa, Takatsuki Red Cross Hospital; Hiroshi Kanamaru, Takeshi Soda, Kitano Hospital; Takayuki Hashimura, Kaoru Murakami, Kansai Electric Power Hospital; Mutsushi Kawakita, Takashi Matsuoka, Kobe City Medical Center General Hospital; Noriyuki Ito, Yosuke Shimizu, Nishikobe Medical Center; Yoji Taki, Jun Watanabe, Toyoka Public Hospital; Hiroshi Iwamura, Ayumu Matsuda, Hmeji Medical Center; Kazuhiro Okumura, Hiroaki Kawanishi, Tenri Hospital; and Tadashi Hayashi, Masahiro Tamaki, Japanese Red Cross Society Wakayama Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
About this article
Cite this article
Terada, N., Akamatsu, S., Okada, Y. et al. Factors predicting efficacy and adverse effects of enzalutamide in Japanese patients with castration-resistant prostate cancer: results of retrospective multi-institutional study. Int J Clin Oncol 21, 1155–1161 (2016). https://doi.org/10.1007/s10147-016-1004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1004-y