Abstract
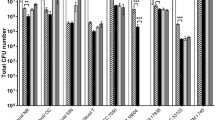
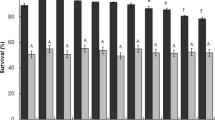
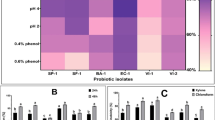
Synbiotics have been intensively studied recently to improve gut health of humans and animals. The success of synergistic synbiotics depends on the compatibility of the prebiotic and probiotic components. Certain plant extracts possess both antimicrobial and prebiotic properties representing a potential use in combination with probiotics to improve the gut health. Here, we coined the term “prophybiotics” to describe this combined bioactivity. The current study aimed to select prebiotics that are preferred as an energy source and antimicrobial plant extracts which do not inhibit the growth, of six strains of lactic acid bacteria (LAB namely; Lactiplantibacillus plantarum, Lacticaseibacillus casei, Limosilactobacillus reuteri, Lacticaseibacillus rhamnosus, Leuconostoc mesenteroides, and Pediococcus pentosaceus) in-vitro to identify compatible combinations for potential synbiotic/prophybiotic use, respectively. Their growth kinetics were profiled in the presence of prebiotics: Inulin, Raffinose, and Saccharicterpenin with glucose, as the control, using carbohydrate free MRS broth media. Similarly, their growth kinetics in MRS broth supplemented with turmeric, green tea, and garlic extracts at varying concentrations were profiled. The results revealed the most compatible pairs of prebiotics and LAB. Turmeric and garlic had very little inhibitory effect on the growth of the LAB while green tea inhibited the growth of all LAB in a dose-dependent manner. Therefore, we conclude that turmeric and garlic have broad potential for use in prophybiotics, while the prebiotics studied here have limited use in synbiotics, with these LAB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A healthy gut microbiome is largely responsible for maintaining innate immunity, gut barrier functioning as well as direct and indirect exclusion of pathogens (Diaz Carrasco et al. 2019). The use of probiotics, prebiotics, and synbiotics (prebiotics + probiotics) to improve gut health of humans and animal species has been studied and reported intensively in literature (as reviewed by Yadav et al. 2022). Lactic acid bacteria (LAB) have been intensively studied and are widely used as probiotics with a wide range of beneficial properties (Ljungh and Wadström 2006). Indeed, many species of LAB are listed in the updated list of qualified presumption of safety (QPS) recommended microorganisms by European Food Safety Authority (EFSA Biohaz Panel (EFSA Panel on Biological Hazards) et al. 2023) indicating the potential use of LAB in humans and animals safely.
According to the latest consensus statement by international scientific association for probiotics and prebiotics (ISAPP), based on the components and their functional role, synbiotics are divided into two main categories namely: complementary and synergistic (Swanson et al. 2020). A complementary synbiotic is a mixture of a probiotic and prebiotic chosen to act individually to improve gut health of the host while a synergistic synbiotic is a combination of live microorganisms which have beneficial effects on a host and a substrate which can selectively stimulate the growth and activity of the chosen microorganism. The selection of components in a complementary synbiotic is relatively easier given the fact that they are expected to affect the host individually. However, the selection of a components for a synergistic synbiotic requires more carefully planned studies to select the most compatible prebiotic that effectively improves the growth and functioning of the probiotic of choice (Quintero et al. 2022). Therefore, careful screening of components in a synbiotic development is crucial for its successful application. Thus, the first objective of the current study was to determine the effect of commercial oligosaccharide-based prebiotics (Inulin, Raffinose, and Sachcharicter penin) on the growth of six strains of LAB to identify the best combinations for potential synbiotic use in terms of in-vitro growth.
As an innovative approach to synergistic synbiotics with oligosaccharide based prebiotics, plant-based second-generation synbiotics have been reviewed by Sharma and Padwad (2020). Here, the authors address the problems of using conventional oligosaccharides including, supporting growth of non-beneficial bacteria, inconsistent observations in clinical endpoints, and lack of inherent bioactivity for improving the gut health (Bindels et al. 2015; Krumbeck et al. 2016) and propose plant-based polyphenolic substrates as a better companion for probiotics in synergistic synbiotics. Among these plant-based bioactives, turmeric (Curcuma longa) (Scazzocchio et al. 2020), green tea (Camellia sinensis) (Jung et al. 2017), and garlic (Allium sativum) (Chen et al. 2019) and did not inhibit the growth of L. acidophilus (Ilham et al. 2018), L. acidophilus A001F8, L. rhamnosus A001G8, L. paracasei A002C5, L. plantarum A003A7, and L. casei A003D4 (Kim et al. 2020). In addition to that, previous literature has also shown that turmeric in combination with Lactobacillus probiotics resulted in enhanced antimicrobial activity (Kim et al. 2020) and anti-allergic inflammatory activity (Yazdi et al. 2020) while improving poultry production parameters (Kinati et al. 2022). These studies along with our current results indicate that turmeric may be a potential candidate to use in combination with Lactobacillus species without affecting bacterial growth for potential prophybiotic application.
On the other hand, existing literature has shown that green tea modulates the composition of intestinal microbiota to improve overall gut health (Chen et al. 2019), while green tea in combination with probiotics reduced the high-fat-diet-induced inflammation in mice (Axling et al. 2012) and hepatorenal syndrome in rat model (Al-Okbi et al. 2019), indicating that green tea is an excellent candidate for prophybiotic application. However, our results demonstrate that supplementation of green tea extracts at higher doses can inhibit the growth of the LAB strains used. In contrast, Story et al. (2009) found that the growth of L. acidophilus and L. gasseri was increased even at higher concentrations of green tea supplementation. Moreover, several studies have reported that the count of Lactobacillus starter cultures in yoghurt is increased with green tea supplementation (Lim 2017; Marhamatizadeh et al. 2013; Najgebauer-Lejko 2014). Interestingly, Janiak et al. (2018) claim that the variation of effects of green tea on probiotic growth could be due to the composition of polyphenolic compounds. The authors reported that the catechins (monomeric flavan-3-ols) help to modulate the growth of microorganisms more selectively than the polymeric fraction in green tea. Proanthocyanidins will inhibit microbial growth more generally and efficiently. Therefore, these findings highlight the importance of performing the individual growth curves for selected probiotic strains with a particular green tea extract when selecting the combinations for potential prophybiotic application as the LAB strains used in the current study showed sensitivity to green tea at higher concentrations.
Our results indicated that garlic extract did not inhibit the growth of most LAB strains while it displayed prebiotic effects on some strains. Interestingly, garlic has been reported to have prebiotic effects particularly on Lactobacillus species (Lu et al. 2021; Sunu et al. 2019; Sutherland et al. 2009) and Bifidobacterium species (Zhang et al. 2013). However, some contrasting results have also been found in the literature. Altuntas and Korukluoglu (2019) and Booyens and Thantsha (2013) observed that garlic extracts display antimicrobial effects on L. acidophilus and Bifidobacterium species. However, in the latter study, the inhibition of probiotic growth by fresh garlic extracts (crushing garlic cloves) was significantly higher than that of garlic powder extract. The authors suggest that it is possibly due to the presence of more active allinase enzymes in fresh cloves when compared with the powdered garlic which will produce more allicin (the active antimicrobial compound) during the extraction process. Since powdered garlic has been used in the current study, it is possible that the allicin content in our garlic extract was less than that of the study of Booyens and Thantsha which resulted in inhibition of probiotics. However, in the same study, it was shown that the sensitivity of different probiotics to garlic extract varied. Therefore, it is also possible that the strains that we have tested in the current study are more resistant to antimicrobial effects of garlic. Therefore, it is imperative to focus on the content of the antimicrobial compounds in the phytobiotics when screening for potential prophybiotic combinations. Therefore, growth curve analysis of probiotics in each case is required to develop successful potential prophybiotics.
It is also important to highlight that the effect of the supplementation of these prebiotics and plant extracts may be different in different strains of the same LAB species owing to wide metabolic differences within the strains of LAB species. Nonetheless, considering the results of the strains used in the current study, prophybiotic formulation seemed promising as plant extracts used in the current study did not inhibit the LAB studied in two out of three species. Therefore, it shows the potential to use a mixture of these LAB along with plant extracts (turmeric or garlic) to optimize the beneficial effects on the gut health of the host. However, as the LAB were very selective in their ability to exploit the commercial prebiotics as their energy source, use of a mixture of LAB with commercial prebiotics, as a synergistic symbiotic, might not be possible due to this selectivity. Nevertheless, the main constraint of prophybiotic formulations is the differences among different cultivars or different extraction systems in terms of bioactive composition. Therefore, we suggest that more future research is necessary to elucidate the potential of prophybiotic formulation, minimizing these constraints.
Conclusion
Garlic and turmeric extracts displayed non-inhibitory effects for all LAB strains studied indicating their potential to use in prophybiotic formulations in the future. Nevertheless, the commercial prebiotics displayed the potential as an energy substrate limited only to particular LAB indicating a limited use of these prebiotics in synergistic symbiotic formulation with the LAB studied.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adamczak A, Ożarowski M, Karpiński TM (2020) Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 13(7):153. https://doi.org/10.3390/ph13070153
Al-Okbi YS, Mohamed AD, Hamed EST, Abd El Khalek AB, Mohammed ES (2019) Role of probiotic mixture with and without green tea extract in prevention of hepatorenal syndrome in rat model. Pak J Biol Sci 22(1):21–27. https://doi.org/10.3923/pjbs.2019.21.27
Altuntas S, Korukluoglu M (2019) Growth and effect of garlic (Allium sativum) on selected beneficial bacteria. Food Sci Technol 39:897–904. https://doi.org/10.1590/fst.10618
Anggraeni AA (2022) Mini-review: the potential of raffinose as a prebiotic. IOP Conf Ser: Earth and Environmental Science 980(1):012033. https://doi.org/10.1088/1755-1315/980/1/012033
Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Ström K, Ahrné S, Holm C, Molin G, Berger K (2012) Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Matab 9:105. https://doi.org/10.1186/1743-7075-9-105
Bhatwalkar SB, Mondal R, Krishna SBN, Adam JK, Govender P, Anupam R (2021) Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front Microbiol 12. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2021.613077
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12(5):Article 5. https://doi.org/10.1038/nrgastro.2015.47
Booyens J, Thantsha MS (2013) Antibacterial effect of hydrosoluble extracts of garlic (Allium sativum) against Bifidobacterium spp. And Lactobacillus acidophilus. Afr J Microbiol Res 7(8):669–677. https://doi.org/10.5897/AJMR12.1906
Chen X, Zhu W, Liu X, Li T, Geng Z, Wan X (2019) The growth performance, meat quality, and gut bacteria of broilers raised with or without antibiotics and green tea powder. J Appl Poult Res 28(3):712–721. https://doi.org/10.3382/japr/pfz023
Chen K, Nakasone Y, **e K, Sakao K, Hou DX (2020) Modulation of allicin-free garlic on gut microbiome. Molecules 25(3):682. https://doi.org/10.3390/molecules25030682
Diaz Carrasco JM, Casanova NA, Fernández Miyakawa ME (2019) Microbiota, gut health and chicken productivity: what is the connection? Microorganisms 7(10):374. https://doi.org/10.3390/microorganisms7100374
EFSA Biohaz Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A et al (2023) Scientific Opinion on the update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA J 21(1):7747. https://doi.org/10.2903/j.efsa.2023.7747
Gopal J, Muthu M, Paul D, Kim DH, Chun S (2016) Bactericidal activity of green tea extracts: the importance of catechin containing nano particles. Sci Rep 6(1):Article 1. https://doi.org/10.1038/srep19710
Ilham LA, Herla R, Dwi S, DewiRestuana S (2018) Antimicrobial activity of turmeric leaf extract against Escherichia coli, Staphylococcus aureus, Shigella dysenteriae, and Lactobacillus acidophilus. IOP Conf Ser: Earth and Environmental Science 205(1):012048. https://doi.org/10.1088/1755-1315/205/1/012048
Janiak MA, Amarowicz R, Rostek D (2018) Influence of catechin fraction and high molecular fraction from green tea extract on Lactobacillus, Bifidobacterium and Streptococcus strains. Nat Prod Commun 13(6) https://doi.org/10.1177/1934578X1801300615
Jhj-lavipan-2021-pl_A4.pdf. http://jhj.pl/files/karty_prod/Ulotki_2021/jhj-lavipan-2021-pl_A4.pdf. Accessed July 14 2023
Jung ES, Park HM, Hyun SM, Shon JC, Liu KH, Hwang JS, Lee CH (2017) Modulation of gut microbiome by green tea diet and its correlation with metabolic changes. Drug Metab Pharmacokinet 32(1 Supplement):S62. https://doi.org/10.1016/j.dmpk.2016.10.248
Kim J, Kim H, Jeon S, Jo J, Kim Y, Kim H (2020) Synergistic antibacterial effects of probiotic lactic acid bacteria with curcuma longa rhizome extract as synbiotic against Cutibacterium acnes. Appl sci 10(24):Article 24. https://doi.org/10.3390/app10248955
Kinati C, Ameha N, Girma M, Nurfeta A (2022) Effective microorganisms, turmeric (Curcuma longa), and their combination on performance and economic benefits in broilers. Heliyon 8(6):e09568. https://doi.org/10.1016/j.heliyon.2022.e09568
Krumbeck JA, Maldonado-Gomez MX, Ramer-Tait AE, Hutkins RW (2016) Prebiotics and synbiotics: dietary strategies for improving gut health. Curr Opin Gastroenterol 32(2):110. https://doi.org/10.1097/MOG.0000000000000249
Lim ES (2017) Effect of green tea supplementation on probiotic potential, physico-chemical, and functional properties of yogurt. Misainmurhag Hoiji 53(2):103–117. https://doi.org/10.7845/kjm.2017.7035
Liu J, Zeng T, Du X, Li G, Yu Y, Lu L, Li C (2016) Effects of water supplemented with Saccharicterpenin and photosynthetic bacteria on egg production, egg quality, serum immunoglobulins, and digestive enzyme activities of ducks. Can J Anim Sci 96(3):397–403. https://doi.org/10.1139/cjas-2015-0139
Liu G, Zheng J, Wu X, Jia G, Zhao H, Chen X, Wu C, Wang J (2019) Effects of saccharicterpenin on antioxidant status and urinary metabolic profile of rats. Anim Nutr 5(2):191–195. https://doi.org/10.1016/j.aninu.2018.09.003
Ljungh A, Wadström T (2006) Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7(2):73–89
Lu X, Li N, Zhao R, Zhao M, Cui X, Xu Y, Qiao X (2021) In vitro prebiotic properties of garlic polysaccharides and its oligosaccharide mixtures obtained by acid hydrolysis. Front Nutr 8. https://www.frontiersin.org/articles/https://doi.org/10.3389/fnut.2021.798450
Marhamatizadeh MH, Ehsandoost E, Gholami P (2013) The influence of green tea (Camellia sinensis L.) extract on characteristic of probiotic bacteria in milk and yoghurt during fermentation and refrigerated storage. Int J Farming Allied Sci 2(17):599–606. http://www.ijfas.com/wp-content/uploads/2013/09/599-606.pdf
Najgebauer-Lejko D (2014) Effect of green tea supplementation on the microbiological, antioxidant, and sensory properties of probiotic milks. Dairy Sci Technol 94(4):327–339. https://doi.org/10.1007/s13594-014-0165-6
Peng H, ShanShan Z, XueWu Z, TingTing Z, ChuanBin X (2011) Effects of dietary saccharicterpenin on mucosal structure of small intestine in Gushi chickens. Chin J Anim Nutr 23(11):2016–2023. https://www.cabdirect.org/cabdirect/abstract/20123074110
Quintero GDF, Kok CR, Hutkins R (2022) The future of synbiotics: rational formulation and design. Front Microbiol 13. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2022.919725
Scazzocchio B, Minghetti L, D’Archivio M (2020) Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin. Nutrients 12(9):2499. https://doi.org/10.3390/nu12092499
Sharma R, Padwad Y (2020) Plant-polyphenols based second-generation synbiotics: emerging concepts, challenges, and opportunities. Nutrition 77:110785. https://doi.org/10.1016/j.nut.2020.110785
Smialek M, Burchardt S, Koncicki A (2018) The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp. - Field study. Res Vet Sci 118:312–316. https://doi.org/10.1016/j.rvsc.2018.03.009
Smialek M, Kaczorek E, Szczucińska E, Burchardt S, Kowalczyk J, Tykałowski B, Koncicki A (2019) Evaluation of Lactobacillus spp. and yeast based probiotic (Lavipan) supplementation for the reduction of Salmonella Enteritidis after infection of broiler chickens. Pol J Vet Sci 22(1):5–10. https://doi.org/10.24425/pjvs.2018.125616
Story EN, Azcarate-Peril A, Klaenhammer TR, Harris GK (2009) Effect of green tea and EGCG on the growth rate and cell density of the probiotic bacteria Lactobacillus acidophilus and Lactobacillus gasseri. FASEB J 23(S1):719.11-719.11. https://doi.org/10.1096/fasebj.23.1_supplement.719.11
Sunu P, Sunarti D, Mahfudz LD, Yunianto VD (2019) Prebiotic activity of garlic (Allium sativum) extract on Lactobacillus acidophilus. Vet World 12(12):2046–2051. https://doi.org/10.14202/vetworld.2019.2046-2051
Sutherland J, Miles M, Hedderley D, Li J, Devoy S, Sutton K, Lauren D (2009) In vitro effects of food extracts on selected probiotic and pathogenic bacteria. Int J Food Sci Nutr 60(8):717–727. https://doi.org/10.3109/09637480802165650
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17(11):Article 11. https://doi.org/10.1038/s41575-020-0344-2
Teferra TF (2021) Possible actions of inulin as prebiotic polysaccharide: a review. Food Front 2(4):407–416. https://doi.org/10.1002/fft2.92
Wu XD, Chen Y, Huang W (2017) A Perspective on the application of pro-/synbiotics in clinical practice. Front Microbiol 8:866. https://doi.org/10.3389/fmicb.2017.00866
Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK (2022) Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol 106:505–521. https://doi.org/10.1007/s00253-021-11646-8
Yazdi GF, Soleimanian-Zad S, Worm VDE, Folkerts G (2019) Turmeric extract: potential use as a prebiotic and anti-inflammatory compound? Plant Foods Hum Nutr 74(3):293–299. https://doi.org/10.1007/s11130-019-00733-x
Yazdi GF, Zakeri A, Ark, IV, Leusink-Muis, T, Braber S, Soleimanian-Zad, S, Folkerts G (2020) Crude turmeric extract improves the suppressive effects of Lactobacillus rhamnosus GG on allergic inflammation in a murine model of house dust mite-induced asthma. Front. immunol 11:1092. https://www.frontiersin.org/articles/https://doi.org/10.3389/fimmu.2020.01092
Zhang N, Huang X, Zeng Y, Wu X, Peng X (2013) Study on prebiotic effectiveness of neutral garlic fructan in vitro. Food Sci Hum Wellness 2(3):119–123. https://doi.org/10.1016/j.fshw.2013.07.001
Acknowledgements
We acknowledge IC Lab, Krakow, Poland, for providing the Hidex Sense microplate reader and JHJ sp z o.o., Poland, for providing lactic acid bacteria for our experiment. We also thank Ovobime project funded by National Science Centre, Poland, and Kaesler Nutrition GmbH, Cuxhaven, Germany for supplying us the prebiotics and plant extracts (turmeric and green tea) for this study.
Funding
This research was carried out under the funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 955374.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ramesha N. Wishna Kadawarage. The first draft of the manuscript was written by Ramesha N. Wishna Kadawarage. Funding acquisition, supervision, review, and editing was by Rita M. Hickey and Maria Siwek. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wishna-Kadawarage, R.N., Jensen, M., Powałowski, S. et al. In-vitro screening of compatible synbiotics and (introducing) “prophybiotics” as a tool to improve gut health. Int Microbiol 27, 645–657 (2024). https://doi.org/10.1007/s10123-023-00417-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00417-2