Abstract
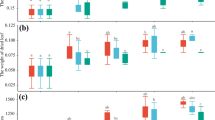
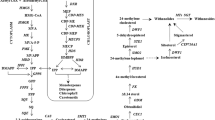
Endophytes have been shown to play a crucial role in determining the fitness of host plant during their association, yet the cross-functional effect of endophytes of one plant on another plant remains largely uncharacterized. In this study, we attempt to analyze the effect of native endophytes of Coleus forskohlii (Phialemoniopsis cornearis (SF1), Macrophomina pseudophaseolina (SF2), and Fusarium redolens (RF1), isolated from stem and root parts) on plant growth and secondary metabolite enhancement in medicinal plant Andrographis paniculata, and aromatic plants Pelargonium graveolens and Artemisia pallens. Here, we report, endophytic treatments with SF2 (21%) and RF1 (9%) in A. paniculata resulted in significant enhancement of andrographolide along with plant primary productivity. Correspondingly, application of fungal endophytes RF1, SF1, and SF2 significantly improved the plant growth (11 to 40%), shoot weight (28 to 34%), oil content (44 to 58%), and oil yield (72 to 122%) in P. graveolens. Interestingly, treatment of A. pallens with three fungal endophytes resulted in significant enhancement of plant productivity and oil content (12 to 80%) and oil yield (32 to 139%). Subsequently, the endophyte treatments RF1 and SF1 enhanced davanone (13 to 22%) and ethyl cinnamate (11 to 22%) content. However, SF2 endophyte-treated plants did not show any improvement in ethyl cinnamate content but enhanced the content of davanone (10%), a signature component of davana essential oil. Overall, results depict cross-functional role of native endophytes of C. forskohlii and repurposing of functional endophytes for sustainable cultivation of economically important medicinal and aromatic crops.




Similar content being viewed by others
References
Ahlawat A, Saxena A, Abdin MZ (2015) Piriformospora indica elicitation of withaferin A biosynthesis and biomass accumulation in cell suspension cultures of Withania somnifera. Symbiosis 69:37–46
Alok A, Shukla V, Pala Z, Kumar J, Kudale S, Desai N (2016) In vitro regeneration and optimization of factors affecting Agrobacterium mediated transformation in Artemisia Pallens, an important medicinal plant. Physiol Mol Biol Plants 22:261–269
Arnold EA (2007) Understanding the diversity of foliar endophytic fungi: progress, challenges and frontiers. Fungal Biol Rev 21:51–66
Bhagavathy GV, Velazquez Nieves GM, Webb MZ, Chauhan KR (2015) Arthropod deterrents from Artemisia pallens (Davana oil) components. Nat Prod Commun 10:1335–1336
Boukhris M, Nasri-Ayachi MB, Mezghani I, Bouaziz M, Boukhris M, Sayadi S (2013) Trichomes morphology, structure and essential oils of Pelargonium graveolens L’Hér. (Geraniaceae). Ind Crop Prod 50:604–610
Clevenger JF (1928) Apparatus for the determination of volatile oil. J Am Pharm Assoc 17:345–349
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant associated bacteria. Crit Rev Microbiol 21:1–18
Cui JL, Wang YN, Jiao J, Gong Y, Wang JH, Wang ML (2017) Fungal endophyte-induced salidroside and tyrosol biosynthesis combined with signal cross-talk and the mechanism of enzyme gene expression in Rhodiola crenulata. Sci Rep 7:12540
Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M, Tuteja N, Johri AK, Varma A (2012) The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal Behav 7:103–112
Dutta SC, Neog B (2016) Accumulation of secondary metabolites in response to antioxidant activity of turmeric rhizomes co-inoculated with native arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Sci Hortic 204:179–184
Faeth SH, Fagan WF (2002) Fungal endophytes: common host plant symbionts but uncommon mutualists. Integr Comp Biol 42(2):360–368
Fakhro A, Andrade-Linares DR, von Bargen S, Bandte M, Buttner C, Grosch R, Schwarz D, Franken P (2010) Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 20:191–200
Gautam AK (2014) The genera Colletotrichum: an incitant of numerous new plant diseases in India. Journal on New Biological Reports 3:9–21
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Harman GE, Doni F, Khadka RB, Uphoff N (2019) Endophytic strains of Trichoderma increase plants' photosynthetic capability. J Appl Microbiol. https://doi.org/10.1111/jam.14368
Hiruma K, Gerlach N, Sacristán S, Nakano RT, Hacquard S, Kracher B, Neumann U, Ramírez D, Bucher M, O'Connell RJ, Schulze-Lefert P (2016) Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165:464–474
Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, Al-Rawahi A, Al-Khiziri S, Ullah I, Ali L, Jung HY, Lee IJ (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52(8):689–695
Kong Z, Mohamad OA, Deng Z, Liu X, Glick BR, Wei G (2015) Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ Sci Pollut Res 22:12479–12489
Mallavarapu GR, Kulkarni RN, Baskaran K, Rao L, Ramesh S (1999) Influence of plant growth stage on the essential oil content and composition in Davana (Artemisia pallens Wall.). J Agric Food Chem 47:254–258
Mastan A, Bharadwaj RKB, Kushwaha RK, Vivek Babu CS (2019a) Functional fungal endophytes in Coleus forskohlii regulate labdane diterpene biosynthesis for elevated forskolin accumulation in roots. Microb Ecol 78:914–926. https://doi.org/10.1007/s00248-019-01376-w
Mastan A, Rane D, Dastager SG, Vivek Babu CS (2019b) Development of low-cost plant probiotic formulations of functional endophytes for sustainable cultivation of Coleus forskohlii. Microbiol Res 227:126310. https://doi.org/10.1016/j.micres.2019
Mukherjee AA, Kandhare AD, Rojatkar SR, Bodhankar SL (2017) Ameliorative effects of Artemisia pallens in a murine model of ovalbumin-induced allergic asthma via modulation of biochemical perturbations. Biomed Pharmacother 94:880–889
Nakhare S, Garg SC (1991) Anthelmintic activity of the essential oil of Artemisia pallens Wall. Anc Sci Life 10:185–186
Okhuarobo A, Falodun JE, Erharuyi O, Imieje V, Falodun A, Langer P (2014) Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its phytochemistry and pharmacology. Asian Pac J Trop Dis 4:213–222
Pala Z, Shukla V, Alok A, Kudale S, Desai N (2016) Enhanced production of an anti-malarial compound artesunate by hairy root cultures and phytochemical analysis of Artemisia pallens Wall. 3 Biotech 6:182
Pandey K (2005) Management of Meloidogyne incognita in Artemisia pallens with bio-organics. Phytoparasitica 33:304–308
Pandey SS, Singh S, Babu CS, Shanker K, Srivastava NK, Shukla AK, Kalra A (2016) Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep 6:26583
Prasad D, Singh KP, Kumar J (2008) Root rot and wilt complex of rose-scented Geranium (Pelargonium graveolens) in Uttarakhand. Indian Phytopath 61(3):365–366
Prasad R, Kamal S, Sharma PK, Oelmueller R, Varma A (2013) Root endophyte Piriformospora indica DSM 11827 alters plant morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. J Basic Microbiol 53:1016–1024
Rajani M, Shrivastava N, Ravishankara MN (2000) A rapid method for isolation of andrographolide from Andrographis paniculata nees (kalmegh). Pharm Biol 38:204–209
Ruikar AD, Misar AV, Jadhav RB, Rojatkar SR, Mujumdar AM, Puranik VG, Deshpande NR (2011) Sesquiterpene lactone, a potent drug molecule from Artemisia pallens wall with anti-inflammatory activity. Arzneimittelforschung 61:510–514
Saxena G, Banerjee S, Laiq-ur-Rahman et al (2007) Rose-scented geranium (Pelargonium sp.) generated by Agrobacterium rhizogenes mediated Ri-insertion for improved essential oil quality. Plant Cell Tiss Organ Cult 90:215
Schaefer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, Scholz U, Pons-Kuehnemann J, Sonnewald S, Sonnewald U, Kogel KH (2009) Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J 59:461–474
Sharma G, Agarwal V (2013) Marked enhancement in the artemisinin content and biomass productivity in Artemisia annua L. shoots co-cultivated with Piriformospora indica. World J Microbiol Biotechnol 29:1133–1138
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2:587. https://doi.org/10.1186/2193-1801-2-587
Shukla V, Pala Z, Alok A, Desai N (2015) Screening of different Artemisia spp. from western ghats of Maharashtra for an antimalarial compound-artemisinin. Am J Plant Sci 06:1619–1632
Strehmel N, Mönchgesang S, Herklotz S, Krüger S, Ziegler J, Scheel D (2016) Piriformospora indica stimulates root metabolism of Arabidopsis thaliana. Int J Mol Sci 17
Subramoniam A, Pushpangadan P, Rajasekharan S, Evans DA, Latha PG, Valsaraj R (1996) Effects of Artemisia pallens wall. on blood glucose levels in normal and alloxan-induced diabetic rats. J Ethnopharmacol 50:13–17
Tanaka A, Makino A (2009) Photosynthetic research in plant science. Plant Cell Physiol 50(4):681–683
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Verma S, Varma A, Rexer K, Hassel A, Kost G, Sarbhoy A, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90:896–903
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17:10754–10773
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156
Acknowledgments
The authors express their sincere thanks to the Director, CSIR- Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for providing his support, encouragement, and necessary facilities during the course of investigation.
Funding
This work was supported by BSC0117 and BSC203 (XII Five-Year Plan Network Project) from Council of Scientific and Industrial Research (CSIR), India. AM greatly acknowledges to Department of Science and Technology (DST–INSPIRE) New Delhi, India, for providing the financial support in the form of fellowship.
Author information
Authors and Affiliations
Contributions
AM and CSV designed the research and performed the work. Data was analyzed by AM, CSV and wrote the manuscript. CH provided the seed material and analyzed field data. KVNSS, ANK, and JKK estimated the oil samples by GC analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This research article does not comprise any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
CIMAP Publication Communication Number
CIMAP/PUB/2019/Mar/12.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Suppl Fig. 1 Function-fungal endophytes (RF1, SF1, and SF2) of Coleus forskohlii. Suppl Fig. 2 Effect of fungal endophytes (RF1, SF1 and SF2) on plant growth response of P. graveolens under field conditions. The control plants in plot (a), RF1 (b), SF1 (c), and SF2 (d). The images (plots) represent the field view of endophytes treated P. graveolens plants (n = 16) at the time of harvesting. Suppl Fig. 3 GC analysis of geranium essential oil composition. The chromatograms represent control and endophyte treatments of P. graveolens. (a) Control, (b) RF1, (c) SF1, and (d) SF2. (DOCX 5265 kb)
Rights and permissions
About this article
Cite this article
Mastan, A., Vivek Babu, C.S., Hiremath, C. et al. Treatments with native Coleus forskohlii endophytes improve fitness and secondary metabolite production of some medicinal and aromatic plants. Int Microbiol 23, 345–354 (2020). https://doi.org/10.1007/s10123-019-00108-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-019-00108-x