Abstract
Background
Reliability of mismatch repair proteins and microsatellite instability assessment is essential in order to define treatment strategy and identify candidates to immune checkpoint inhibitors in locally advanced gastroesophageal carcinoma. We evaluated the concordance of deficient mismatch repair (dMMR) and microsatellite instability-high (MSI-H) status between endoscopic biopsies and surgical specimens.
Methods
Consecutive patients with resectable gastric or gastroesophageal junction adenocarcinoma classified as MSI-H/dMMR by polymerase chain reaction (PCR) or immunohistochemistry (IHC) and operated at three referral Institutions were included. The primary endpoint was the rate of concordance between biopsy and surgical samples. If needed, central revision by IHC/PCR was performed by specialized pathologists from coordinating Institutions.
Results
Thirteen (19.7%) out of 66 patients showed discordant MSI-H/dMMR results in the original pathology reports. In most cases (11, 16.7%) this was due to the diagnosis of proficient mismatch repair status on biopsies. Among the ten cases available for central review, four were due to sample issues, four were reclassified as dMMR, one case showed dMMR status but was classified as microsatellite stable by PCR, one was linked to misdiagnosis of endoscopic biopsy by the local pathologist. Heterogeneity of mismatch repair proteins staining was observed in two cases.
Conclusions
Available methods can lead to conflicting results in MSI-H/dMMR evaluation between endoscopic biopsies and surgical samples of gastroesophageal adenocarcinoma. Strategies aiming to improve the reliability of assessment should be primarily focused on the optimization of tissue collection and management during endoscopy and adequate training of dedicated gastrointestinal pathologists within the multidisciplinary team.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal adenocarcinoma is an aggressive malignancy, characterized by poor prognosis when treated with surgery alone in the locally advanced setting [1]. Different multimodal strategies proved to be effective in improving patient outcomes in randomized trials, and multidisciplinary management of these patients is currently regarded as the preferred approach [2]. In particular, perioperative chemotherapy is considered the standard treatment in Europe, with neoadjuvant chemoradiotherapy being an alternative option in selected patients with Siewert I–II gastroesophageal junction (GEJ) tumours [2,3,4].
In the absence of reliable molecular biomarkers, candidates for a multimodality management are selected mainly on the basis of clinical staging. The only exception is represented by deficient mismatch repair proteins (dMMR) status, a peculiar molecular alteration found in approximately 10% of patients with locally advanced gastroesophageal adenocarcinoma and leading to microsatellite instability-high (MSI-H) [5]. MSI-H/dMMR tumours are characterized by better prognosis when treated with surgery alone, and seem to derive little if any benefit from multimodality treatment, even though uncertainties still exist (particularly when docetaxel-based triplet chemotherapy is administered perioperatively) [6, 7]. Therefore, the optimal management of locally advanced GEJ and gastric (G) cancer patients with MSI-H/dMMR tumours is still under debate [8, 9].
Along with its prognostic role, MSI-H/dMMR status is also a predictive biomarker of higher sensitivity to immune checkpoint inhibitors (ICIs) in different tumour types [10], including gastroesophageal cancer [11]. Evidence of benefit from ICIs in this subset is now moving to earlier disease stages, paving the way toward a chemotherapy-free approach for molecularly selected cases. The NEONIPIGA study reported significant activity of nivolumab plus ipilimumab among 32 patients with MSI-H/dMMR cT2-4 cNx cM0 GEJ/G adenocarcinoma, with a 58.6% rate of pathologic complete responses (pCRs) among R0 resected patients [12]. These data were replicated in the INFINITY trial with the combination of durvalumab plus tremelimumab: in the first cohort of 18 patients with cT2-4 cNx cM0, a 60% pCR rate among the 15 evaluable patients was reported, and the benefit of immunotherapy appeared to be dependent on primary tumour stage (pCR in cT4 vs. cT2-3: 17% vs. 89%) [13]. On the light of these promising results, non-operative management in MSI-H/dMMR GEJ/G tumours obtaining a clinical complete response to neoadjuvant immunotherapy is under investigation [13].
On the basis of these data, a reliable assessment of the MSI-H/dMMR status in endoscopic diagnostic biopsies is recommended in updated guidelines since this is of extreme relevance in both routine practice and clinical trials [14, 15]. However, evidence in colorectal cancer shows that up to 10% of MSI-H/dMMR cases may be misdiagnosed at local assessment by polymerase chain reaction (PCR) or immunohistochemistry (IHC), which partially explains the primary resistance to ICIs [16]. Moreover, in advanced gastroesophageal cancer, resistance to ICIs has also been found to be associated with heterogenous microsatellite or MMR status, too, with microsatellite stable (MSS)/mismatch repair proficient (pMMR) areas of the tumour driving disease progression [17, 18]. This rises some concern in resectable disease, when the initial diagnosis is often based on limited tumour tissue collected by endoscopic biopsies.
In the present study, we aimed to assess the rate of concordance of MSI-H/dMMR status between diagnostic biopsies and surgical samples as determined by PCR and/or IHC in a consecutive real-world series of resectable gastroesophageal adenocarcinomas.
Methods
Study population
Consecutive patients with clinical stage I–III GEJ/G adenocarcinoma who underwent surgery (with or without preoperative treatment) at three referral Italian institutions from October 2005 to December 2021 were screened for eligibility. All patients with evidence of MSI-H/dMMR status based on routine testing as per everyday practice, performed on surgical specimens and/or on diagnostic biopsies, were included in the final analysis. Most of the original diagnostic biopsies and MSI-H/dMMR status evaluation were performed in other hospitals than the three referral centres. For each case, the original haematoxylin–eosin stains and the original pathology report were jointly reviewed by two expert gastrointestinal pathologists (C.U., M.F.).
For each patient included, the following clinicopathological data were collected: age at diagnosis, gender, site of origin, performance status, clinical stage at diagnosis, pathological stage, Human Epidermal Growth Factor Receptor 2 (HER2) expression (and amplification, if performed), Epstein-Barr Virus (EBV) status (assessed by Epstein-Barr encoding region [EBER] in situ hybridization), Programmed Death-Ligand 1 (PD-L1) combined positive score (CPS) result, time to metastases, type and number of metastatic sites, and systemic treatments received (neoadjuvant, adjuvant and metastatic setting).
All information regarding human material was managed using anonymous numerical codes, and all samples were handled in compliance with the Declaration of Helsinki and current laws, ethics and regulations. The protocol was approved by the Ethics Committee of Veneto Institute of Oncology – IRCCS (approval number 2020/140 dated 16/Nov/2020).
MSI/MMR status assessment
IHC data for MMR proteins expression were obtained both on diagnostic biopsies and resected specimens. In case of debatable interpretation, or of discordant results between the two samples/diagnoses, PCR testing was performed, as confirmatory test.
IHC was performed by using the following: (i) fully automated BenchMark ULTRA system platform (Roche-Ventana Medical Systems, Tucson, AZ, USA) while the antigen–antibody reaction was visualized using the Optiview DAB IHC VENTANA Detection Kit; (ii) automated platform Autostainer Link 48 (Dako, Carpinteria, CA, USA) while the antigen–antibody reaction was visualized using the EnVision FLEX kit with diaminobenzidine as chromogen.
The dMMR status was assessed by testing MSH2, MSH6, MLH1 and PMS2, and samples were defined as dMMR when PMS2 or MSH6 or both proteins of the heterodimer resulted negative. Nuclear positive staining in stromal cells and lymphocytes or normal mucosa was used as internal positive controls [19].
MSI molecular testing was performed after PCR amplification by using (i) EasyPGX melting analysis and (ii) the Titano kit (Diatech Pharmacogenetics).
Endpoints and statistics
The primary endpoint was the rate of concordance between biopsy and surgical samples in defining MSI-H/dMMR status, both for PCR and IHC: concordance was defined as MSI-H/dMMR status confirmed by any technique (IHC, PCR) in both tissue samples. Secondary endpoints were represented by the rate of heterogeneous microsatellite or MMR status in different tumour areas and the rate of misdiagnosis of MSI-H/dMMR status.
The retrospective analyses of clinical data were performed using a prospective database. Given the exploratory nature of the study, no formal sample size calculations were elaborated.
Fisher’s exact test and Pearson’s χ2 test were applied to evaluate the association of concordant and discordant cases on biopsy tissue with baseline clinicopathological characteristics. Overall survival (OS) was calculated from the time of initial diagnosis (or the date of evidence of disease recurrence, as appropriate) until death due to any cause. Disease-free survival (DFS) was calculated from the time of surgery to progression/relapse (distant and/or local), death, or last follow-up. Survival curves were performed on the overall population using the Kaplan–Meier method and compared using the log-rank test. Statistical significance was set at p < 0.05 for a two-sided test. All statistical analyses were performed using Medcalc version 14.8.1 (Ostend, Belgium).
Results
Patient characteristics
After revision of the internal datasets of 484 resected gastroesophageal adenocarcinomas tested for MMR/MSI status, a total of 66 patients were finally included in the study (Fig. 1). Characteristics of the population are presented in Table 1. As expected, most of the patients (39, 59.1%) had 70 years or more (median age: 71 years, range: 40–90), and 83.3% of primary lesions were located in the gastric body or antrum. Only 12 (1.5%) samples were tested for PD-L1 expression, and all but one had CPS ≥ 5. One (1.5%) of the samples was classified as mucinous adenocarcinoma, whereas mucinous features or signet-ring cells were recognized in other 5 (7.6%) cases.
Nineteen patients (28.8%) received preoperative treatment as follows: 11 were treated with oxaliplatin-based triplets (9 FLOT and 2 EOX regimen), 5 with cisplatin-based doublets, 2 with ICIs and 1 with concomitant cisplatin plus 5-fluorouracil and radiation. Additional data about DFS, OS, patterns of recurrence and clinical outcomes after recurrence are presented as Supplementary material.
Concordance of MSI/dMMR status between paired biopsy and surgical samples
The results of the initial analysis are reported in Table 2 and Fig. 2A. All but one patient had their surgical samples tested for MSI/MMR status: 56 samples were assessed by IHC, 3 samples by PCR and 6 samples by both methods, whereas 1 resection specimen was not tested due to quality failure (no tumour cells in the available sample). Sixty-three out of 65 (96.9%) evaluable cases were reported as MSI-H/dMMR cases, 1 was reported as pMMR by IHC (limited tumour cellularity of the available sample made PCR analysis not feasible) and 1 case presented heterogenous microsatellite status due to heterogeneity in MLH1-PMS2 staining across different tumour areas.
Results of initial MSI/MMR testing (A) and central revision of discordant cases (B). IHC immunohistochemistry, MMR mismatch repair (dMMR deficient MMR, pMMR proficient MMR), MSI microsatellite instability (MSI-H microsatellite instability high), MSS microsatellite stable, PCR polymerase chain reaction
With regard to diagnostic endoscopies, the exact number of biopsy punches collected during the procedure was specified in 51 reports (77.3%): the median number of biopsies that contained tumour cells was 3 (range: 1–7). A pMMR status by IHC was defined in the original pathology report for 11 cases (16.7%). In the other 55 cases, MSI-H/dMMR was diagnosed by IHC (n = 47), PCR (n = 5) or both (n = 3). Among MSI-H/dMMR cases, 2 presented heterogeneity in MLH1-PMS2 staining at IHC (associated with heterogeneity in corresponding surgical sample in 1 case; Fig. 3). Only 1 out of 13 (7.7%) discordant cases was a mucinous adenocarcinoma with signet-ring cell component at pathology assessment. With regard to the number of endoscopic biopsies, data were available for 44 (83.0%) concordant and 7 (53.8%) discordant cases: the median/mean number of biopsies collected was 3/3.2 (range: 1–7) and 3/3.9 (range: 1–7), respectively. Four out of 13 (30.8%) patients with discordant MMR/MSI status had received preoperative treatment (3 with chemotherapy containing a platinum plus fluoropyrimidine with or without docetaxel, and 1 with ICIs). No association was reported between discordant MSI/MMR status and clinical (tumour location, previous preoperative treatment) or pathological (mucinous histology or features, histology, grading, stage) characteristics (all p > 0.05; data not shown).
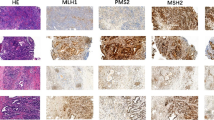
Heterogeneity of mismatch repair (MMR) status at immunohistochemistry (IHC). A representative case of phenotypically mixed type gastric cancer, characterized also by a heterogeneity of MMR status at IHC. The adenocarcinoma presented an area of low-grade tubular adenocarcinoma coexisting with a poorly cohesive histotype (haematoxylin and eosin, H&E). MLH1 and PMS2 IHC were retained in the poorly cohesive counterpart and were lost in the tubular adenocarcinoma
In the entire cohort, 13 cases (19.7%) showed different results in MSI/MMR status between biopsies and surgical specimens in the original pathology reports (biopsy classified as pMMR and surgical specimen MSI-H/dMMR in 11 cases [16.7%]: biopsy classified as MSI-H/dMMR and surgical specimen pMMR in 1 case [1.5%]; biopsy and surgical specimen classified as dMMR by IHC but MSS by PCR on biopsy specimen in 1 case [1.5%]) (Table 2 and Fig. 2A).
In 3 (23.1%) discordant cases (MSS/pMMR on the biopsy and MSI-H/dMMR on surgical specimen) MSI/MMR status was not centrally reassessable by either PCR or centrally performed IHC due to slides and tissue blocks unavailability.
The other 10 (76.9%) discordant cases were centrally reviewed by two independent experienced pathologists of the referral institutions (Table 2 and Fig. 2B).
In 4 (30.8%) cases, the discordant diagnosis of pMMR status on biopsies was imputable to sample issues by central revision (inadequate fixation time in 2 cases, staining artefacts in 1 case and limited tumour tissue in 1 case). In such cases, tumour cells showed mild or focal staining, associated with moderate/strong positive controls staining. Two cases have been retested by PCR, while available material was judged as insufficient in the other 2 cases.
Four (30.8%) cases (3 biopsies and 1 surgical specimen) were reclassified as dMMR by IHC after central review. Two biopsy cases were originally misinterpreted as MSS/pMMR on biopsy because the only loss of PMS2 was not considered sufficient for a dMMR status by the local pathologist and were defined by central IHC evaluation as dMMR (as it was the corresponding surgical specimen). In 1 of these cases molecular analysis showed MSS status in both samples. A biopsy case of a poorly cohesive carcinoma was misinterpreted as positive due to the limited amount of neoplastic cell component within stromal MMR-positive cells. The surgical specimen suffered of pre-analytical issues that led to a very faint MMR positivity misinterpreted as negative from the local pathologist.
Discrepancy between IHC and molecular assessment (dMMR and MSS, respectively) was evident in 1 (7.7%) biopsy sample, but suboptimal quality of tumour tissue for MSI analysis had to be considered (cellularity: 25%).
Pathological misdiagnosis was evident in 1 (7.7%) initially defined pMMR biopsy: tissue reassessment showed non-invasive high-grade dysplasia with no concurrent infiltrative/adenocarcinomatous component of the lesion.
To summarize, we reported discordant results for MSI/MMR status between biopsy and surgical specimen in 13 out of 66 patients included in the analysis (19.7%). This discrepancy was mainly due to pMMR status on biopsies of otherwise MSI-H/dMMR surgical samples (the only patient with pMMR disease on surgical sample had confirmed MSI-H/dMMR disease at biopsy by PCR and IHC). Main identifiable reasons behind discordant results relied on quality of biopsy specimen (6 cases, 46.2%) and misinterpretation of IHC results for MMR assessment performed in “spoke” surgical pathology units (4 cases, 30.8%). Globally, after central review, 5 matched samples could be re-classified as concordant dMMR, while 5 could not be re-assessed due to tissue inadequacy. Therefore, in our population sensitivity of locally performed IHC in correctly identifying dMMR on biopsy tissue was 81.2% (54/66), and it reached at least 87.9% (58/66) when IHC was evaluated by dedicated pathologists in a reference center for gastrointestinal cancers. Notably, intratumour heterogeneity of MMR proteins staining was observed in two cases (3% of the entire cohort) on endoscopic biopsies, and in one case the same staining pattern was observed in the surgical sample.
Discussion
In the past years, raising evidences support the negative impact of MSI-H/dMMR on chemosensitivity in GEJ/G adenocarcinoma and suggest limited or no benefit from preoperative combination chemotherapy in this subgroup of patients [6]. In addition, multiple studies are assessing the efficacy of neoadjuvant ICIs among patients with MSI-H/dMMR GEJ/G adenocarcinoma, with promising results [12, 13]. In the meantime, the refinement of microsatellite status diagnosis on biopsy samples is required to properly choose the correct treatment strategy.
In the present study among patients with resectable GEJ/G adenocarcinoma, approximately one out of five cases showed discordant results in MSI/MMR analyses between biopsies and surgical specimens. Notably, the main cause of this discrepancy was the limited quantity and quality of tumour sampling by endoscopic biopsies as well as the misinterpretation of IHC analyses for MMR status. Moreover, in 12 out of 13 discordant cases, the initial diagnosis on biopsy specimen reported MSS/pMMR status. When assessed by a dedicated pathologist, 5 out of 5 reviewed biopsies were defined as MSI-H/dMMR, coherently with the surgery sample, reducing the discordance between the two specimens to 12.1% (8 out of 66). The percentage of misclassification might be even higher, but unavailability of tumour tissue from 3 biopsy samples limited the possibility of central revision of microsatellite status in these cases.
Even though results of the current analysis seem to reassure about the reliability of widespread used techniques such as PCR and IHC for the assessment of MSI/MMR status in gastroesophageal cancer patients when used by experienced and trained pathologists, we believe that some issues should be considered in translating these results into research context and routine practice.
Our results suggest that misinterpretation of IHC staining results could be a risk, particularly in case of poor quality or limited cellularity of biopsy samples, when the analysis is performed by non-dedicated gastrointestinal pathologists. In such cases, technical and preanalytical issues could further impair reliability of MMR evaluation, as observed in 46.2% of discordant biopsy samples in our study [20, 21]. The false negative result of IHC testing performed on biopsy could result in loss of chances for treatment with ICIs within clinical trials [12, 13], while the benefit of perioperative chemotherapy might be reduced in such cases. The pathologist should recognize patterns associated with pre-analytical problems, including fixation and cautery artifacts, cold ischemia, and staining artefacts. If such artefacts prevent the pathologist from performing an adequate evaluation, MMR status should be regarded as equivocal or not assessable, and the staining should be repeated on a different tissue block, if available. Furthermore, the evaluation of the biomarker should be performed exclusively on the invasive carcinoma and the entire tumour area should be evaluated. As demonstrated by one discordant case in our series, the dysplastic component should not be taken into account when assessing biomarkers’ status [22]. However, making a proper distinction between non-invasive and invasive components can be difficult and the morphologic diagnosis of dysplasia is burdened by a certain degree of interobserver variability [23]. Another potential pitfall is that poorly cohesive tumour cells of gastroesophageal adenocarcinomas may be difficult to distinguish from stromal and inflammatory cells on IHC. In these cases, a haematoxylin–eosin or cytokeratin slide may be helpful in the evaluation of the entire tumour area.
Notably, 4 patients with discordant MMR/MSI status had received prior neoadjuvant treatment (3 with chemotherapy, 1 with ICIs). Chemotherapy as well as chemoradiotherapy has been associated with abnormal staining patterns of MMR proteins [24, 25]. In our series, tissue inadequacy prevented revision in 3 of such cases, whereas discrepancy in 1 case was explained by initial misinterpretation of MMR status IHC (corrected after central revision). These data suggest that adequate interventions in terms of pathologist education as well as quality initiatives on tissue collection could partly overcome potential pitfalls related to preoperative treatment.
We reported MSI/MMR heterogeneity in 3% of the cases included in the study, and this could be of concern when MSI/MMR status is used as the key driver of patient management. Intratumour heterogeneity is a well-known challenging issue in HER2 status assessment in gastroesophageal adenocarcinoma [26], and data about differential expression among distinct tumour areas of other potential biomarkers (such as claudin 18.2 [27, 28] and PD-L1 [29]) have been reported. Intratumor heterogeneity of MMR/MSI status may lead to discordant interpretations between matched biopsies and surgical specimens due to the presence of different subpopulations of cells within the tumour having distinct MMR/MSI status or due to the coexistence of two adjacent pMMR and dMMR synchronous neoplasms [30]. Here we confirmed that even MSI/MMR status might be subject to intratumour heterogeneity, as observed by other authors [17, 18, 31]. Such heterogeneity might contribute to increase complexity of MSI assessment and might result in significant diagnostic challenges when biopsy samples are limited. Nonetheless, in our 2 heterogeneous cases a dMMR status by IHC in both biopsy and surgical samples was reported, suggesting that diagnostic challenges could be overcome by the availability of representative tissue from different tumour areas. Indeed, the impact of microsatellite status heterogeneity in terms of prognostic and predictive significance is still unclear. Previous reports highlighted a link between heterogeneity and resistance to ICIs in the palliative setting [17, 18], but the role in locally advanced disease needs to be clarified. In particular, whether these patients derive the same benefit from neoadjuvant ICIs as the overall population of MSI-H/dMMR cases is unknown: since no patient with heterogeneous MMR proteins staining was treated with ICIs in our series, we could not answer this question in the present study. When assessing biomarkers’ status, the pathologist must be aware of the biological background of molecular intratumor heterogeneity and of the possible clinical implications, and thus, should always mention the presence of intratumor heterogeneity in the pathology report.
In order to overcome the aforementioned issues, we advocate the implementation of two key improvements into everyday practice. First, adequate sampling of different areas of gastroesophageal tumours could increase the accuracy of MSI/MMR status assessment, overcoming the challenges due to intratumour heterogeneity and biopsy quality failure. Since biopsy samples were judged insufficient or inadequate for further analyses in a not negligible percentage of cases, we believe that careful discussion is needed with endoscopists within the multidisciplinary teams, in order to improve diagnostic sampling of primary gastroesophageal lesions for molecular analyses. On top of technical problems related to preanalytical variables that are assessed in detail elsewhere [20, 21], no definitive recommendation about sampling collection is available for microsatellite status assessment. Information about the number of endoscopic biopsies collected was available for 77.3% of the patients, and only 6 (9.1%) cases in the entire series had 6 or more punches available: indeed, we cannot firmly establish the recommended number of biopsies to adequately assess MMR status on the basis of our data. Current guidelines suggest the collection of at least 6 biopsies for HER2 evaluation [32] and endoscopy is generally performed before other imaging staging procedures: therefore, it seems reasonable to propose that at least 6 punches should be obtained in all GEJ/G cancer patients. This approach is supported also by published literature for biomarkers that will hopefully enter the clinics in the near future, such as claudin 18.2 expression [28]. Second, adequate training of pathologists dealing with biomarkers evaluation in gastrointestinal tumours is mandatory. Lessons learned in decades of anti-HER2 strategies in breast cancer demonstrate that the performance of local HER2 testing improved over time, reaching a high concordance rate with central assessment in more recent years [33]. Greater accuracy and increased predictive value of centrally assessed HER2 status has been demonstrated also in gastroesophageal cancer, confirming the need for effective educational and coaching initiatives particularly among pathologists from low-volume centres [34]. In our series, MMR status had been assessed on non-infiltrative dysplastic lesion in 1 case, again validating the conclusion that the quality of molecular analyses is strictly related to the reliability of pathology assessment [22]. Taken together, all these data underline the need for dedicated educational plans and are reassuring about the potential usefulness of quality initiatives in diagnostic pathology.
To conclude, our large series of MSI-H/dMMR resectable gastroesophageal cancer cases identifies potential discrepancy in microsatellite status between biopsy and corresponding surgical samples in a small but significant percentage of patients. Known and manageable reasons behind this discrepancy rely on misdiagnosis and tumour heterogeneity. Standardised protocols for biopsy collection of suspected gastroesophageal lesions as well as training programmes for dedicated gastrointestinal pathologists could help minimizing the risks of misdiagnosis and increasing the chances of benefitting patients with ICIs.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48. https://doi.org/10.1016/S0140-6736(20)31288-5.
Fornaro L, Vasile E, Aprile G, Goetze TO, Vivaldi C, Falcone A, Al-Batran SE. Locally advanced gastro-oesophageal cancer: recent therapeutic advances and research directions. Cancer Treat Rev. 2018;69:90–100. https://doi.org/10.1016/j.ctrv.2018.06.012.
Reynolds JV, Preston SR, O’Neill B, Lowery MA, Baeksgaard L, Crosby T, et al. Neo-AEGIS (Neoadjuvant Trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): final primary outcome analysis. J Clin Oncol. 2023;41(suppl 4):295–295. https://doi.org/10.1200/JCO.2023.41.4_suppl.295.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57. https://doi.org/10.1016/S0140-6736(18)32557-1.
Han S, Chok AY, Peh DYY, Ho JZ, Tan EKW, Koo SL, et al. The distinct clinical trajectory, metastatic sites, and immunobiology of microsatellite-instability-high cancers. Front Genet. 2022;13:933475. https://doi.org/10.3389/fgene.2022.933475.
Pietrantonio F, Miceli R, Raimondi A, Kim YW, Kang WK, Langley RE, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–400. https://doi.org/10.1200/JCO.19.01124.
Kohlruss M, Grosser B, Krenauer M, Slotta-Huspenina J, Jesinghaus M, Blank S, et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: role of Epstein-Barr virus infection and high- and low-microsatellite instability. J Pathol Clin Res. 2019;5:227–39. https://doi.org/10.1002/cjp2.137.
Lordick F. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020;21:203. https://doi.org/10.1016/S1470-2045(20)30012-7.
Smyth EC. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020;21:204. https://doi.org/10.1016/S1470-2045(20)30025-5.
Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33:929–38. https://doi.org/10.1016/j.annonc.2022.05.519.
Pietrantonio F, Randon G, Di Bartolomeo M, Luciani A, Chao J, Smyth EC, Petrelli F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6:100036. https://doi.org/10.1016/j.esmoop.2020.100036.
André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2023;41:255–65. https://doi.org/10.1200/JCO.22.00686.
Pietrantonio F, Raimondi A, Lonardi S, Murgioni S, Cardellino GG, Tamberi S, et al. INFINITY: a multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023. https://doi.org/10.1200/JCO.2023.41.3_suppl.358.
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC, ESMO Guidelines Committee. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–20. https://doi.org/10.1016/j.annonc.2022.07.004.
Businello G, Angerilli V, Lonardi S, Bergamo F, Valmasoni M, Farinati F, et al. Current molecular biomarkers evaluation in gastric/gastroesophageal junction adenocarcinoma: pathologist does matter. Updates Surg. 2023;75:291–303. https://doi.org/10.1007/s13304-022-01330-5.
Cohen R, Hain E, Buhard O, Guilloux A, Bardier A, Kaci R, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol. 2019;5:551–5. https://doi.org/10.1001/jamaoncol.2018.4942.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. https://doi.org/10.1126/science.aan6733.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58. https://doi.org/10.1038/s41591-018-0101-z.
Fassan M, Scarpa A, Remo A, De Maglio G, Troncone G, Marchetti A, et al. Current prognostic and predictive biomarkers for gastrointestinal tumors in clinical practice. Pathologica. 2020;112:248–59. https://doi.org/10.32074/1591-951X-158.
Bateman AC. DNA mismatch repair protein immunohistochemistry—An illustrated guide. Histopathology. 2021;79:128–38. https://doi.org/10.1111/his.14367.
Malapelle U, Parente P, Pepe F, De Luca C, Cerino P, Covelli C, et al. Impact of pre-analytical factors on MSI test accuracy in mucinous colorectal adenocarcinoma: a multi-assay concordance study. Cells. 2020;9:2019. https://doi.org/10.3390/cells9092019.
Angerilli V, Lonardi S, Farinati F, Savarino E, Bergamo F, Fassan M. Mismatch repair status and gastro-oesophageal dysplasia: need for a dedicated gastrointestinal pathologist? Histopathology. 2022;80:1138–40. https://doi.org/10.1111/his.14647.
Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–78. https://doi.org/10.1053/hupa.2001.23510.
Vilkin A, Halpern M, Morgenstern S, Brazovski E, Gingold-Belfer R, Boltin D, et al. How reliable is immunohistochemical staining for DNA mismatch repair proteins performed after neoadjuvant chemoradiation? Hum Pathol. 2014;45:2029–36. https://doi.org/10.1016/j.humpath.2014.07.005.
Kuan SF, Ren B, Brand R, Dudley B, Pai RK. Neoadjuvant therapy in microsatellite-stable colorectal carcinoma induces concomitant loss of MSH6 and Ki-67 expression. Hum Pathol. 2017;63:33–9. https://doi.org/10.1016/j.humpath.2017.02.003.
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–84. https://doi.org/10.1007/s10120-014-0402-y.
Coati I, Lotz G, Fanelli GN, Brignola S, Lanza C, Cappellesso R, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer. 2019;121:257–63. https://doi.org/10.1038/s41416-019-0508-4.
Pellino A, Brignola S, Riello E, Niero M, Murgioni S, Guido M, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med. 2021;11:1095. https://doi.org/10.3390/jpm11111095.
Zhou KI, Peterson B, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;26:6453–63. https://doi.org/10.1158/1078-0432.CCR-20-2085.
von Loga K, Woolston A, Punta M, Barber LJ, Griffiths B, Semiannikova M, et al. Extreme intratumour heterogeneity and driver evolution in mismatch repair deficient gastro-oesophageal cancer. Nat Commun. 2020;11:139. https://doi.org/10.1038/s41467-019-13915-7.
Wang X, Jiang K, Hu Y, Zhao X, Yin L, Diao X, et al. An exploration of gastric cancer with heterogeneous mismatch repair status. Virchows Arch. 2023;482:517–23. https://doi.org/10.1007/s00428-023-03506-9.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:446–64. https://doi.org/10.1200/JCO.2016.69.4836.
Pfitzner BM, Lederer B, Lindner J, Solbach C, Engels K, Rezai M, et al. Clinical relevance and concordance of HER2 status in local and central testing-an analysis of 1581 HER2-positive breast carcinomas over 12 years. Mod Pathol. 2018;31:607–15. https://doi.org/10.1038/modpathol.2017.171.
Haffner I, Schierle K, Raimúndez E, Geier B, Maier D, Hasenaue J, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol. 2021;39:1468–78. https://doi.org/10.1200/JCO.20.02761.
Acknowledgements
This research was partially supported by Veneto Institute of Oncology IOV-IRCCS research program BIOV19LOUPAK. MF is supported by grants from the Italian Health Ministry/Veneto region research program NET-2016–02363853 and AIRC 5 per mille 2019 (ID. 22759 program). FP is supported by grants from AIRC IG 23624.
Author information
Authors and Affiliations
Contributions
LF, SL, CU and MF conceptualized and designed the study. All the authors collected and analysed data. LF, SL, SC, FN, CU and MF supervised analyses and interpreted data. All the authors drafted the article and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
LF reports personal honoraria as invited speaker from Incyte, Bristol Myers Squibb, Lilly; research funding (to Institution) from MSD, Bristol Myers Squibb, AstraZeneca, Incyte, BeiGene, Astellas, Daiichi Sankyo, Roche; participation in advisory board for MSD, AstraZeneca, Incyte, Taiho, Servier, Daiichi Sankyo, Lilly. SL reports research funding (to Institution) from Amgen, Astellas, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Daichii Sankyo, Hutchinson, Incyte, Merck Serono, Mirati, MSD, Pfizer, Roche, Servier; personal honoraria as invited speaker from Amgen, Bristol-Myers Squibb, Incyte, GSK, Lilly, Merck Serono, MSD, Pierre-Fabre, Roche, Servier; participation in advisory board for Amgen, Astra Zeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Incyte, Lilly, Merck Serono, MSD, Servier. FP reports personal fees from Amgen, Merck-Serono, Pierre-Fabre, Servier, Bayer, MSD, Bristol Myers Squibb, Lilly, AstraZeneca, Astellas, Organon, and research grants from AstraZeneca, Agenus, Incyte and Bristol Myers Squibb. MF has been involved in consulting/advisory roles in Astellas Pharma, Pierre Fabre, MSD, AstraZeneca, Janssen, GlaxoSmithKline, Amgen, Novartis and Roche, and received research funding from Astellas Pharma, QED Therapeutics, Diaceutics and Macrophage Pharma. All other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fornaro, L., Lonardi, S., Catanese, S. et al. Concordance of microsatellite instability and mismatch repair status in paired biopsies and surgical specimens of resectable gastroesophageal adenocarcinoma: time for a call to action. Gastric Cancer 26, 958–968 (2023). https://doi.org/10.1007/s10120-023-01411-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01411-3