Abstract
Background
The aim of this study was to evaluate clinical validity of the S-1 dosage formula based on body surface area (BSA) and creatinine clearance (CLcr) to achieve the target area under the concentration–time curve of 5-FU, which we had developed and refined in each prospective pharmacokinetic study.
Methods
The recommended dose determined by the refined formula was assessed using data of the SPIRITS (S-1 vs. S-1 plus cisplatin [SP]) and the G-SOX (SP vs. S-1 plus oxaliplatin [SOX]) trials. Nine hundred and thirty-eight patients in these trials were classified into three groups according to their actual S-1 starting doses compared with the recommended doses (under-dosed, <recommended dose; equal-dosed, =recommended dose; over-dosed, >recommended dose).
Results
The patients in the under-dosed group in both trials showed similar tendencies: male, younger, higher BSA, and higher CLcr. The incidence of any grade neutropenia was significantly greater in the over-dosed group compared with the equal-dosed group in the S-1 and the SOX arms. The hazard ratios (HR) of overall survival (OS) (under-dosed vs. equal-dosed) were 1.361 (S-1 arm), 1.259 (SP arm) in the SPIRITS trial, and 1.381 (SOX arm), 0.999 (SP arm) in the G-SOX trial. Multivariate analysis in all the patients demonstrated that OS of the over-dosed group was equivalent (HR 1.002, 95% confidence interval [CI] 0.850–1.182, p = 0.980) and that of the under-dosed group was inferior (HR 1.267, 95% CI 1.005–1.597, p = 0.045) to the equal-dosed group.
Conclusions
It is suggested that the refined S-1 dosage formula can recommend optimal dose in terms of safety and efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
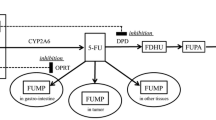
S-1 is an oral antitumor agent containing tegafur (a prodrug of 5-fluorouracil [5-FU]), 5-chloro-2,4-dihydroxypyridine (CDHP) and potassium oxonate. CDHP inhibits dihydropyrimidine dehydrogenase (DPD), which is a rate-limiting enzyme catabolizing 5-FU, leading to maintenance of high 5-FU concentration after S-1 administration. Potassium oxonate is specifically distributed in the epithelium of intestine and inhibits the phosphorylation of 5-FU, to reduce gastrointestinal toxicity [1,2,3]. Since S-1 was approved for gastric cancer in Japan in 1999, it has been widely used in Asia (TS-1®; Taiho Pharmaceutical, Tokyo, Japan) and Europe (Teysuno®; Nordic Group, Hoofddorp, Netherlands). The Asian approved dose of S-1 in its monotherapy is 80 mg/m2/day (as tegafur) for 4 weeks followed by rest for 2 weeks [4, 5]. The European approved dose of S-1 is 50 mg/m2/day for 3 weeks followed by rest for 1 week in combination with cisplatin 75 mg/m2 every 4 weeks [6]. S-1-containing regimens have been reported to improve clinical outcomes in various cancers, such as gastric [7,8,9,10,11], non-small cell lung [12,13,14], colorectal [15, 16], breast [17], pancreatic [18], and biliary tract cancers [19].
As >50% of CDHP is excreted in the urine [20], renal dysfunction has been reported to increase the exposure of CDHP, leading to excessive inhibition of DPD which results in a sustained high concentration of 5-FU in not only animal model [21] but also in cancer patients as we reported [22]. Therefore, S-1 is contraindicated to patients with severe renal impairment (creatinine clearance [CLcr] < 30 mL/min). A post-marketing survey on S-1 demonstrated that the incidences of adverse reactions were high in patients with impaired renal function [23]. The US Food and Drug Administration and the European Medicines Agency provide the guidance for industry requiring to assess the pharmacokinetics of a new drug in subjects with impaired renal function during the development and to propose the dose adjusted for them in the prescribing information. However, there is no similar guidance in Asia including Japan; thus, reliable recommendation on S-1 dose reduction for the Asian approved dose of 80 mg/m2/day in patients with renal dysfunction has not been established. Therefore, we conducted a pharmacokinetic study of 16 cancer patients with varied renal function and developed an S-1 dosage formula based on CLcr and body surface area (BSA) by population pharmacokinetic (PPK) analysis to achieve the target area under the concentration–time curve (AUC) of 5-FU: Recommended daily dose = target AUC of 5-FU × (21.9 + 0.375 × CLcr) × BSA [22]. Furthermore, we proposed the nomograms for the recommended daily dose of S-1 to achieve the target AUC of 1447.8 ng·h/mL [20] for Asian approved dose and 1177.2 ng·h/mL [24] for European approved dose, respectively, according to individual BSA and CLcr by taking into account the approved dosage strengths in tablets/capsules (20 and 25 mg, as tegafur, in Asia; 15 and 20 mg, as tegafur, in Europe). Thereafter, we conducted a pharmacokinetic study of 30 cancer patients with various renal functions to evaluate the S-1 recommended dose determined by the nomogram derived from our previously developed formula. The PPK analysis from the combined data of 46 patients in our two studies identified that sex was a significant covariate for 5-FU clearance. Hence, the formula was refined including sex as an additional factor: Recommended daily dose = target AUC of 5-FU × (14.5 + 8.23 × SEX [0 for female and 1 for male] + 0.301 × CLcr) × BSA [25]. The revised nomograms also derived from the refined formula showing the recommended daily doses of S-1 separately for male and female patients were proposed for both Asian and European approved doses. These refined S-1 dosage formula and nomograms are expected to be useful for clinical practice to achieve the target AUC ensuring efficacy and safety of S-1.
Because S-1 is a key drug for treating advanced gastric cancer, several prospective phase 3 trials of chemotherapy regimens containing S-1 have been conducted, contributing to the establishment of standard treatments for advanced gastric cancer. The SPIRITS trial demonstrated that S-1 plus cisplatin (SP) was superior to S-1 alone in terms of overall survival (OS) for patients with advanced gastric cancer [9]. The G-SOX trial demonstrated that S-1 plus oxaliplatin (SOX) was as effective as SP for advanced gastric cancer [10]. In this study, we evaluated the clinical validity of our refined S-1 dosage formula and nomograms retrospectively, using the data of both the SPIRITS and G-SOX trials.
Patients and methods
Patients
The subjects of this study were all per-protocol populations whose data were available, comprising 298 patients in the SPIRITS trial (150 patients allocated to S-1 alone and 148 patients allocated to SP) and 640 patients in the G-SOX trial (316 patients allocated to SOX and 324 patients allocated to SP). Eligibility criteria of both trials required adequate renal function. The starting daily dose of S-1 in all arms of the SPIRITS and the G-SOX trials was 80 mg/m2 (<1.25 m2, 80 mg; ≤1.25–<1.50 m2, 100 mg; ≥1.50 m2, 120 mg). In the S-1 alone arm of the SPIRITS trial, S-1 was given for 4 weeks of a 6-week cycle. In the SP arm of the SPIRITS and the G-SOX trials, S-1 was given for 3 weeks of a 5-week cycle and cisplatin at 60 mg/m2 was administered intravenously on day 8 of each cycle. In the SOX arm of the G-SOX trial, S-1 was given for 2 weeks of a 3-week cycle and oxaliplatin at 100 mg/m2 was administered intravenously on day 1 of each cycle. The data of the SPIRITS trial and the G-SOX trial were provided under the agreement with Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan) and Yakult Honsha Co., Ltd. (Tokyo, Japan), respectively. This study was approved by the institutional review board at Keio University School of Medicine.
Study design
The recommended daily S-1 dose, determined by the revised nomograms derived from the refined formula for Asian approved daily dose of 80 mg/m2 (Supplementary Fig. 1), was obtained for each patient according to individual sex, CLcr estimated by Cockcroft–Gault equation and BSA [25] at baseline. Patients were classified into three groups according to their actual starting doses of S-1 compared with the recommended doses by the refined nomograms: under-dosed group, < recommended dose; equal-dosed group, = recommended dose; over-dosed group, > recommended dose). Patient characteristics, treatment delivery including dose intensity, OS, progression-free survival (PFS), and the incidences of treatment-related adverse events (AEs) were compared among the three groups in each arm of the SPIRITS and the G-SOX trials. The definitions of PFS and OS were previously reported in the SPIRITS and the G-SOX trials [9, 10]. Toxicities were graded using the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0 in the SPIRITS trial, and the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 in the G-SOX trial.
Statistical analysis
Categorical data were compared using Fisher’s exact test or the chi-squared test, as appropriate. Quantitative data were analyzed using the unpaired Student’s t-test. Survival was analyzed using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate comparisons of survival time were performed using Cox regression analysis. p < 0.05 was considered significant. Statistical analyses were performed using SPSS version 27.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics
In the 298 patients of the SPIRITS trial, CLcr estimated by Cockcroft–Gault equation ranged from 37.9 to 180.9 mL/min. Among the 150 patients allocated to the S-1 arm, 17 patients (11.3%) were classified into the under-dosed group, 55 patients (36.7%) into the equal-dosed group, and 78 patients (52.0%) into the over-dosed group. Among the 148 patients allocated to the SP arm, 15 patients (10.1%) were classified into the under-dosed group, 45 patients (30.4%) into the equal-dosed group, and 88 patients (59.5%) into the over-dosed group. Baseline characteristics of the patients in the three groups of each arm in the SPIRITS trial are shown in Table 1. The patients in the under-dosed group in both the S-1 and the SP arms showed similar tendencies: male, younger, higher BSA, and higher CLcr. There were no significant differences in Eastern Cooperative Oncology Group (ECOG) performance status (PS), adjuvant chemotherapy, histology, disease status and peritoneal metastasis among the three groups in both arms.
In the 640 patients of the G-SOX trial, CLcr estimated by Cockcroft–Gault equation ranged from 33.7 to 189.2 mL/min. Among the 316 patients allocated to the SOX arm, 56 patients (17.7%) were classified into the under-dosed group, 75 patients (23.7%) into the equal-dosed group, and 185 patients (58.5%) into the over-dosed group. Among the 324 patients allocated to the SP arm, 56 patients (17.3%) were classified into the under-dosed group, 84 patients (25.9%) into the equal-dosed group, and 184 patients (56.8%) into the over-dosed group. Baseline characteristics of the patients in the three groups of each arm of the G-SOX trial are shown in Table 2. Patients in the under-dosed group of both arms were mostly male, younger, higher BSA and higher CLcr as well as patients in the SPIRITS trial. There were also no significant differences in ECOG PS, adjuvant chemotherapy, histology, disease status and peritoneal metastasis among the three groups in both arms.
Treatment delivery
In the SPIRITS trial, the proportions of patients requiring S-1 dose reduction and S-1 cessation were not significantly different between the equal-dosed group and the other groups in either the S-1 arm or the SP arm. Accordingly, actual S-1 dose intensity also did not differ between the equal-dosed group and the other groups. On the other hand, S-1 relative dose intensity (RDI) based on the recommended dose determined by the refined formula was significantly greater in the over-dosed groups of both the S-1 and the SP arms (S-1, 103.9 ± 32.9%; SP, 114.0 ± 32.4%) compared with the equal-dosed group (S-1, 78.3 ± 25.1%; SP, 85.5 ± 19.0%), and the under-dosed group (S-1, 63.1 ± 24.4%; SP, 73.3 ± 15.1%) as shown in Table 3. In addition, S-1 RDI based on the recommended dose determined by the refined formula was significantly less in the under-dosed group (73.3 ± 15.1%) compared with the equal-dosed group (85.5 ± 19.0%) of the SP arm. The proportions of patients requiring cisplatin dose reduction were not significantly different between the equal-dosed group and the other groups of the SP arm; however, cisplatin RDI was significantly greater in the under-dosed group (96.3 ± 10.1%) compared with the equal-dosed group (82.2 ± 25.6%) and the over-dosed group (80.9 ± 24.3%).
In the G-SOX trial, the proportions of patients requiring dose reduction of S-1 were significantly less in the under-dosed groups of both arms (SOX, 8.9%; SP, 25.0%) than those in the equal-dosed groups (SOX, 29.3%; SP, 44.0%) as shown in Table 4. In the SOX arm, the proportion of patients requiring dose reduction of S-1 was also significantly greater in the over-dosed group (48.6%) than that in the equal-dosed group (29.3%). S-1 RDIs based on the recommended dose determined by the refined formula were significantly less in the under-dosed group of both the SOX and the SP arms (SOX, 67.2 ± 15.2%; SP, 67.2 ± 11.0%) compared with the equal-dosed groups (SOX, 81.4 ± 15.4%; SP, 79.8 ± 15.3%). In addition, S-1 RDIs based on the recommended dose determined by the refined formula were significantly greater in the over-dosed group of both the SOX and the SP arms (SOX, 97.7 ± 23.8%; SP, 102.7 ± 28.3%) compared with the equal-dosed group (SOX, 81.4 ± 15.4%; SP, 79.8 ± 15.3%) and the under-dosed group (SOX, 67.2 ± 15.2%; SP, 67.2 ± 11.0%). RDI of oxaliplatin was significantly greater in the under-dosed group (85.5 ± 17.5%) than that in the equal-dosed group (77.5 ± 21.6%) of the SOX arm. RDIs of oxaliplatin and cisplatin were also significantly less in the over-dosed groups (73.3 ± 20.2% and 74.5 ± 23.2%) than those in the under-dosed groups (85.5 ± 17.5% and 81.5 ± 18.8%) of the SOX arm and the SP arm, respectively.
Toxicity
Table 3 also shows treatment-related AEs in the three groups of the SPIRITS trial. In the S-1 arm, the incidences of any grade leukopenia and neutropenia were significantly greater in the over-dosed group (50.0 and 57.7%, respectively), compared with the equal-dosed (32.7 and 30.9%, respectively) and under-dosed group (17.6 and 23.5%, respectively). In the SP arm, incidences of anorexia (any grade, grade 3 or 4) were significantly greater in the over-dosed group (88.6 and 45.5%, respectively) compared with the equal-dosed group (68.9 and 26.7%, respectively).
In the SOX arm of the G-SOX trial, incidences of any grade neutropenia and increased creatinine were significantly greater in the over-dosed group (77.3 and 21.1%, respectively), compared with the equal-dosed group (64.0 and 10.7%, respectively) and under-dosed group (44.6 and 8.9%, respectively) as shown in Table 4. Incidences of any grade neutropenia and thrombocytopenia were significantly less in the under-dosed group (44.6 and 62.5%, respectively) than those in the equal-dosed group (64.0 and 86.7%, respectively). Incidence of any grade anemia was significantly greater in the over-dosed group (66.5%) than that in the equal-dosed group (52.0%). In the SP arm of the G-SOX trial, incidence of grade 3 or 4 leukopenia was significantly greater in the over-dosed group (22.8%), compared with the equal-dosed group (11.9%) and under-dosed group (5.4%). Incidence of any grade anemia was also significantly greater in the over-dosed group (83.2%) than that in the equal-dosed group (72.6%).
Efficacy
Kaplan–Meier analysis of survival
The Kaplan–Meier curves of OS and PFS in the three groups of the SPIRITS trial are shown in Fig. 1. In the S-1 arm, the median OS was 12.4 months (95% confidence interval [CI] 9.8–14.9 months) in the over-dosed group, 10.4 months (95% CI 8.3–12.4 months) in the equal-dosed group, and 8.2 months (95% CI 2.6–13.8 months) in the under-dosed group (Fig. 1a). The hazard ratios (HR) were 0.833 (95% CI 0.581–1.192; p = 0.318, over-dosed vs. equal-dosed), 1.361 (95% CI 0.771–2.398; p = 0.289, under-dosed vs. equal-dosed), and 0.613 (95% CI 0.354–1.063; p = 0.081, over-dosed vs. under-dosed). In the SP arm, the median OS was 14.0 months (95% CI 11.0–17.5 months) in the over-dosed group, 13.2 months (95% CI 10.0–16.3 months) in the equal-dosed group, and 10.3 months (95% CI 7.7–12.9 months) in the under-dosed group (Fig. 1c). The HRs were 0.992 (95% CI 0.672–1.464; p = 0.968, over-dosed vs. equal-dosed), 1.259 (95% CI 0.669–2.370; p = 0.476, under-dosed vs. equal-dosed), and 0.754 (95% CI 0.418–1.361; p = 0.349, over-dosed vs. under-dosed). In the S-1 arm, the median PFS was 4.6 months (95% CI 3.8–5.4 months) in the over-dosed group, 4.1 months (95% CI 2.6–5.7 months) in the equal-dosed group, and 1.7 months (95% CI 1.3–2.2 months) in the under-dosed group (Fig. 1b). The HRs were 0.996 (95% CI 0.686–1.445; p = 0.982, over-dosed vs. equal-dosed), 1.477 (95% CI 0.813–2.577; p = 0.209, under-dosed vs. equal-dosed), and 0.664 (95% CI 0.382–1.156; p = 0.148, over-dosed vs. under-dosed). In the SP arm, the median PFS was 7.0 months (95% CI 4.9–9.0 months) in the over-dosed group, 5.4 months (95% CI 2.6–8.1 months) in the equal-dosed group, and 5.8 months (95% CI 2.6–9.0 months) in the under-dosed group (Fig. 1d). The HRs were 0.835 (95% CI 0.568–1.225; p = 0.356, over-dosed vs. equal-dosed), 1.172 (95% CI 0.611–2.247; p = 0.633, under-dosed vs. equal-dosed), and 0.654 (95% CI 0.353–1.211; p = 0.176, over-dosed vs. under-dosed). The under-dosed group tended to show shorter OS and PFS in both the S-1 and the SP arms.
The Kaplan–Meier curves of OS and PFS in the three groups of the G-SOX trial are shown in Fig. 2. In the SOX arm, the median OS was 15.1 months (95% CI 13.7–16.5 months) in the over-dosed group, 13.8 months (95% CI 9.9–17.7 months) in the equal-dosed group, and 10.9 months (95% CI 8.9–12.9 months) in the under-dosed group (Fig. 2a). The HRs were 1.075 (95% CI 0.788–1.466; p = 0.649, over-dosed vs. equal-dosed), 1.381 (95% CI 0.933–2.045; p = 0.107, under-dosed vs. equal-dosed) and 0.779 (95% CI 0.559–1.086; p = 0.141, over-dose vs. under-dose). In the SP arm, the median OS was 13.1 months (95% CI 11.5–14.6 months) in the over-dosed group, 12.8 months (95% CI 9.5–16.0 months) in the equal-dosed group, and 15.5 months (95% CI 10.1–21.0 months) in the under-dosed group (Fig. 2c). The HRs were 1.035 (95% CI 0.773–1.385; p = 0.817, over-dosed vs. equal-dosed), 0.999 (95% CI 0.681–1.466; p = 0.996, under-dosed vs. equal-dosed), and 1.071 (95% CI 0.766–1.495; p = 0.691, over-dosed vs. under-dosed). In the SOX arm, the median PFS was 5.9 months (95% CI 5.2–6.7 months) in the over-dosed group, 7.0 months (95% CI 5.2–8.7 months) in the equal-dosed group, and 4.2 months (95% CI 1.5–6.8 months) in the under-dosed group (Fig. 2b). The HRs were 1.025 (95% CI 0.768–1.368; p = 0.869, over-dosed vs. equal-dosed), 1.330 (95% CI 0.918–1.931; p = 0.132, under-dosed vs equal-dosed), and 0.752 (95% CI 0.545–1.036; p = 0.082, over-dosed vs. under-dosed). In the SP arm, the median PFS was 5.7 months (95% CI 4.9–6.6 months) in the over-dosed group, 5.7 months (95% CI 4.7–6.7 months) in the equal-dosed group, and 6.7 months (95% CI 5.1–8.3 months) in the under-dosed group (Fig. 2d). The HRs were 0.923 (95% CI 0.697–1.222; p = 0.575, over-dosed vs. equal-dosed), 0.885 (95% CI 0.614–1.276; p = 0.512, under-dosed vs. equal-dosed), and 1.064 (95% CI 0.767–1.481; p = 0.710, over-dosed vs. under-dosed). There was a trend for shorter OS and PFS in the under-dosed group of the SOX arm, but not of the SP arm in the G-SOX trial.
Kaplan–Meier survival curves of the three groups classified by the refined formula in the G-SOX trial. a Overall survival and b progression-free survival of the SOX arm; c overall survival and d progression-free survival of the SP arm. SOX S-1 plus oxaliplatin, SP S-1 plus cisplatin, HR hazard ratio, CI confidence interval
There were no significant differences in OS between the younger and the older in both trials when divided by median age (62 years in the SPIRITS trial and 65 years in the G-SOX trial) (Supplementary Figs. 2a and 3a). There were also no significant differences in OS between the males and females when divided by gender (Supplementary Figs. 2b and 3b).
Multivariate analysis of survival
Multivariate analysis including the four factors in the JCOG prognostic index (ECOG PS ≥ 1, number of metastatic sites ≥ 2, no prior gastrectomy, and serum alkaline phosphatase (ALP) level > normal range), other reported two factors (peritoneal dissemination, histology), chemotherapy regimens, and S-1 starting dose classified into three groups by the refined formula in all the patients was performed (Table 5) [26, 27]. This multivariate analysis demonstrated that the classification to “under-dosed” by the S-1 formula was a significant poor prognostic factor compared to “equal-dosed” as well as other well-known prognostic factors (HR 1.267; 95% CI 1.005–1.597; p = 0.045), while the over-dosed and equal-dosed groups showed equivalent survival (HR 1.002, 95% CI 0.50–1.182, p = 0.980). Multivariate analyses of each arm in both trials showed results with similar tendency (supplementary tables 1, 2, 3, 4, 5, and 6).
Discussion
Our goal of develo** an S-1 dosage formula was to recommend the optimal dose of S-1 for improving its efficacy and safety especially in patients with renal dysfunction including the elderly. Hence, we evaluated its clinical validity by comparing toxicity and efficacy between patients receiving S-1 at the recommended dose determined by our refined formula and those receiving at over-dose or under-dose than the recommended dose, using the data of the two prospective randomized phase 3 trials, the SPIRITS and the G-SOX trials, all of whose treatment arms contained S-1 for advanced gastric cancer [9, 10].
With regard to toxicity in the over-dosed group, the incidence of any grade neutropenia in the over-dosed group was significantly greater than those in the equal-dosed group of the S-1 arm and the SOX arm (Tables 3, 4). Therefore, it is expected that the recommended dose determined by the refined formula could improve safety for patients who are classified into the over-dosed group such as female, older, lower BSA and impaired renal function. There was consistent tendency showing more frequency of AEs in the over-dosed groups compared with the equal-dosed groups of SP arm in both SPIRITS and G-SOX trials. However, while the incidence of grade 3 or 4 leucopenia was significantly greater in the over-dosed group than the equal-dosed group in the SP arm of the G-SOX trial, there were no significant differences in the incidences of leucopenia and neutropenia between the over-dosed group and the equal-dosed group in the SP arm of the SPIRITS trial. The overall incidences of AEs and cisplatin dose reduction were lower in the SP arm of the SPIRITS trial compared to the G-SOX trial, although the dose intensities of S-1 and cisplatin were higher in the SPIRITS trial. From the results that renal dysfunction during the whole treatment course was observed less frequently in the SPIRITS trial compared to the G-SOX trial, it was speculated that the management of renal function might be more careful in the SPIRITS trial. It should be kept in mind that renal damage due to cisplatin should be prevented and S-1 dose should be adjusted according to renal function all through the treatment course. Our refined formula and nomograms would be applied to recommend different doses for each patient during treatment course depending on renal function.
In our second study of 30 patients with various renal functions receiving S-1 alone at the doses recommended by our previous developed formula [25], we have reported that three patients (10%) experienced S-1 related grade 3 AEs (neutropenia, anemia, and rash) during the first course. Focusing on the equal-dosed group of the S-1 arm in the SPIRITS trial, the incidences of grade 3 or 4 neutropenia, anemia, and rash were 12.7% (7/55), 12.7% (7/55), and 3.6% (2/55) through the treatment courses. These results suggest that the recommended doses by the refined formula might be associated with less toxicities than those by our previous formula, considering that toxicities were thought to have accumulated along with the treatment course. Clinically, these incidences (about 10% or less) of the grade 3 or 4 toxicities by S-1 alone observed in the equal-dosed group of the SPIRITS trial seem to be acceptable, suggesting the applicability of the recommended dose by the refined formula and nomograms.
As for efficacy, OS and PFS tended to be shorter in the under-dosed group, except the SP arm of the G-SOX trial (Figs. 1, 2). Although differences between groups for any arms in either the SPIRITS or the G-SOX trials were not statistically significant due to the small number of patients, the HRs seem to be clinically meaningful. The prescribing information of S-1 indicates that the dose can be increased up to 150 mg/day according to the tolerability of the individual patient receiving S-1 at started dose; however, dose increase was not allowed in either the SPIRITS or the G-SOX trials [9, 10]. It was suggested that patients classified into the under-dosed group such as male, younger, higher BSA and higher CLcr, who were tolerable for S-1 at started dose, might not achieve the target AUC ensuring safety and efficacy. Therefore, it is expected that the recommended dose determined by the refined formula could result in better efficacy especially for the patients who are classified into the under-dosed group. Considering general clinical management, the dose of patients in the over-dosed group can be reduced to the recommended dose due to the toxicities during the treatment course, which may not reduce the efficacy. In contrast, the dose of patients in the under-dosed group is not increased to the recommended dose even observing its mild toxicities. However, actually, the under-dosed group included male, younger, higher BSA, and higher CLcr, and the prognosis of these patients who originally seemed to have good condition should not be shortened due to under-dose. Both in the SPIRITS trial and the G-SOX trial, age and gender did not affect OS significantly, and JCOG prognostic index whose prognostic impacts were validated in these two clinical trials does not include gender, age, BSA or renal function as an independent prognostic factor [26, 27]. It is suggested that under-dose might worsen the OS rather than difference in background. Higher dose than the standard dose can be proposed for patients classified to the under-dosed group. On the other hand, OS tended to be longer in the over-dosed group in the S-1 arm of the SPIRITS trial (Fig. 1a). It is also speculated that higher AUCs of 5-FU than target AUCs would result in better efficacy in S-1 monotherapy.
In the SP arm of the G-SOX trial, increased serum creatinine has been observed as treatment-related AE in 39.4% (all grades) and 1.8% (grade 3 or 4) [10], which are greater than the 22% (all grades) and 0% (grade 3 or 4) of the SP arm in the SPIRITS trial [9]. The reason why increased serum creatinine was observed more in the G-SOX trial than in the SPIRITS trial would be that one-day hydration without hospitalization was adopted in some cases in the G-SOX trial. Therefore, it is speculated that increasing serum creatinine (decreasing renal function) might result in dose reduction and cessation more frequently even in the under-dosed and equal-dosed groups of the SP arm in the G-SOX trial, meaning conversion of the under-dosed group to the over-dosed group during the treatment course. In addition, both S-1 actual dose intensity and cisplatin RDI were lower in the SP arm of the G-SOX trial than those in the SP arm of the SPIRITS trial (Tables 3, 4). Reduced doses of S-1 and cisplatin might have impacted on reducing efficacy especially in the equal-dosed and over-dosed groups of the SP arm in the G-SOX trial. Not only for the toxicities but also for efficacy, it could be emphasized again that renal damage due to cisplatin should be prevented carefully and S-1 dose should be adjusted according to renal function all through the treatment course.
Our refined formula recommends higher dose of S-1 in male patients than female patients, since our PPK analysis identified that sex was a significant covariate for 5-FU clearance with male patients having higher clearance. In the subgroup analyses of OS in the SPIRITS trial and G-SOX trial, HRs were lower in female than in male in both trials [9, 10], which suggests that the dose of S-1 might not be enough in male. Because the clearance of injected 5-FU has been also reported to be higher in males than that in females [28,29,30], it can be proposed that dose modification of 5-FU depending on sex should be established.
Multivariate analysis including JCOG prognostic index and other well-known prognostic factors suggested two important possibilities (Table 5). First, S-1 dose reduction to the recommended dose determined by the refined formula for over-dosed group would reduce toxicity without decreasing the therapeutic effect. It is proposed that the refined formula can be used as an index of S-1 dosage for patients with renal dysfunction in daily practice, since there is no reliable recommendation nowadays. Second, S-1 dose increment to “equal-dosed” in the under-dosed patients would improve the prognosis although S-1 dose increment might enhance the adverse effects. In the future, the comparative study is needed to verify these risk and benefit.
In recent years, nivolumab has been introduced to the first-line chemotherapy for advanced gastric cancer [31], and S-1 plus docetaxel has become the new standard treatment as adjuvant chemotherapy instead of S-1 alone [32]. It has been reported that these present standard regimens are associated with more severe hematological toxicities compared with the former standard regimens established in the SPIRITS, G-SOX and ACTS-GC trials [7, 9, 10]. In the present clinical practice, it is expected that our refined formula can reduce the toxicities of these present standard chemotherapy regimens in over-dosed patients.
This study has some limitations. The individual changes in renal function during the treatment were not taken into consideration due to retrospective investigation. The recommended doses determined by our refined formula for only Asian approved dose of 80 mg/m2/day were evaluated whereas European approved dose in combination with cisplatin was 50 mg/m2/day.
In conclusion, the clinical validity of our refined S-1 dosage formula based on renal function was retrospectively evaluated using the data of the SPIRITS and G-SOX trials. It is suggested that the refined formula can recommend optimal dose in terms of safety and efficacy for the first-line treatment of advanced gastric cancer.
References
Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–6.
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anti Cancer Drugs. 1996;7:548–57.
Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–9.
Taiho Pharmaceutical Co Ltd. TS-1 Prescribing Information in Japan; TS-1 combination OD tablet. Ver. 25; Jan 2020.
Taiho Pharmaceutical Co Ltd. TS-1 Prescribing Information in Singapore; TS-ONE Capsule. Revised; June 2013.
European Medecines agency science medicines health. Teysuno: EPAR - product Infomartion. 2020. https://www.ema.europa.eu/en/documents/product-information/teysuno-epar-product-information_en.pdf
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8.
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–53.
Nokihara H, Lu S, Mok TSK, Nakagawa K, Yamamoto N, Shi YK, et al. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann Oncol. 2017;28:2698–706.
Yoshioka H, Okamoto I, Morita S, Ando M, Takeda K, Seto T, et al. Efficacy and safety analysis according to histology for S-1 in combination with carboplatin as first-line chemotherapy in patients with advanced non-small-cell lung cancer: updated results of the West Japan Oncology Group LETS study. Ann Oncol. 2013;24:1326–31.
Kubota K, Sakai H, Katakami N, Nishio M, Inoue A, Okamoto H, et al. A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol. 2015;26:1401–8.
Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–86.
Yamada Y, Denda T, Gamoh M, Iwanaga I, Yuki S, Shimodaira H, et al. S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann Oncol. 2018;29:624–31.
Takashima T, Mukai H, Hara F, Matsubara N, Saito T, Takano T, et al. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2016;17:90–8.
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950–8.
Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, et al. Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res. 1999;5:2000–5.
Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor agent in animal model and in patients with impaired renal function. Cancer Chemother Pharmacol. 2002;50:25–32.
Booka E, Imamura CK, Takeuchi H, Hamamoto Y, Gomi D, Mizukami T, et al. Development of an S-1 dosage formula based on renal function by a prospective pharmacokinetic study. Gastric Cancer. 2016;19:876–86.
Nagashima F, Ohtsu A, Yoshida S, Ito K. Japanese nationwide post-marketing survey of S-1 in patients with advanced gastric cancer. Gastric Cancer. 2005;8:6–11.
van Groeningen CJ, Peters GJ, Schornagel JH, Gall H, Noordhuis P, de Vries MJ, et al. Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol. 2000;18:2772–9.
Takeuchi M, Imamura CK, Booka E, Takeuchi H, Mizukami T, Kawakami T, et al. Prospective evaluation and refinement of an S-1 dosage formula based on renal function for the clinical application. Cancer Sci. 2021;112:751–9.
Takahari D, Boku N, Mizusawa J, Takashima A, Yamada Y, Yoshino T, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist. 2014;19:358–66.
Takahari D, Mizusawa J, Koizumi W, Hyodo I, Boku N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer. 2017;20:757–63.
Milano G, Etienne MC, Cassuto-Viguier E, Thyss A, Santini J, Frenay M, et al. Influence of sex and age on fluorouracil clearance. J Clin Oncol. 1992;10:1171–5.
Gusella M, Crepaldi G, Barile C, Bononi A, Menon D, Toso S, et al. Pharmacokinetic and demographic markers of 5-fluorouracil toxicity in 181 patients on adjuvant therapy for colorectal cancer. Ann Oncol. 2006;17:1656–60.
Mueller F, Büchel B, Köberle D, Schürch S, Pfister B, Krähenbühl S, et al. Gender-specific elimination of continuous-infusional 5-fluorouracil in patients with gastrointestinal malignancies: results from a prospective population pharmacokinetic study. Cancer Chemother Pharmacol. 2013;71:361–70.
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47.
Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: Interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–304.
Acknowledgements
The authors thank Taiho Pharmaceutical Co., Ltd. and Yakult Honsha Co., Ltd. for their providing the data of the SPIRITS trial and the G-SOX trial, respectively.
Funding
This work was not supported by any direct or indirect funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Chiyo K. Imamura has received research fund from Otsuka Pharmaceutical Co., Ltd. Hiroya Takeuchi has received scholarship endowments from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Factory Inc, Ono Pharmaceutical Co., Ltd., and Eli Lilly Japan KK. Yusuke Tanigawara has received research funds from Takeda Pharmaceutical Co., Ltd. and Asahi Kasei Pharma Corp.; and consulting fee from Fujimoto Pharmaceutical Co. Yuko Kitagawa has received honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corp., and Otsuka Pharmaceutical Factory Inc, and scholarship endowments from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Factory Inc, Ono Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corp., Yakult Honsha Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd., Tsumura Co., and Medicon Inc. Narikazu Boku has received honoraria from Taiho Pharmaceutical Co., Ltd. and Bristol-Myers Squibb KK, and research funds from Ono Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Booka, E., Imamura, C.K., Takeuchi, M. et al. Evaluation of clinical validity of an S-1 dosage formula based on renal function using data of the SPIRITS and the G-SOX trials. Gastric Cancer 25, 770–782 (2022). https://doi.org/10.1007/s10120-022-01291-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01291-z