Abstract
Respiratory syncytial virus continues to pose a serious threat to the pediatric populations worldwide. With a genomic makeup of 15,200 nucleotides, the virus encodes for 11 proteins serving as envelope spikes, inner envelope proteins, and non-structural and ribonucleocapsid complexes. The fusion (F) and attachment (G) surface glycoproteins are the key targets for neutralizing antibodies. The highly variable G with altered glycosylations and the conformational alternations of F create challenges for vaccine development. The metastable F protein is responsible for RSV-host cell fusion and thus infectivity. Novel antigenic sites were identified on this form following its stabilization and solving its crystal structure. Importantly, site ø displays neutralizing activity exceeding those of post-F-specific and shared antigenic sites, such as site II which is the target for Palivizumab therapeutic antibody. Induction of high neutralizing antibody responses by pre-F immunization in animal models promoted it as a major vaccine candidate. Since RSV infection is more serious at age extremities and in individuals with undermining health conditions, vaccines are being developed to target these populations. Infants below three months of age have a suppressive immune system, making vaccines’ immunogenicity weak. Therefore, a suggested strategy to protect newborns from RSV infection would be through passive immunity of maternal antibodies. Hence, pregnant women at their third trimester have been selected as an ideal target for vaccination with RSV pre-F vaccine. This review summarizes the different modes of RSV pathogenesis and host’s immune response to the infection, and illustrates on the latest updates of vaccine development and vaccination approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Respiratory syncytial virus (RSV) is an enveloped, non-segmented, and negative-strand RNA virus belonging Paramyxoviridae family [1]. It is the most leading cause of lower respiratory tract infections (LRTI) among pediatrics worldwide, with about 33 million cases and about 160,000–190,000 deaths annually, representing 6.7% of all deaths in less than one-year old infants [2]. Hospitalization is common throughout the first five years of age; however, it peaks among three-month-old infants [3]. By the age of three years, the majority of children get infected with RSV [4], then reinfected repeatedly for a lifetime as the host immunity to this virus diminishes over time [5]. Infection with RSV is manifested by airway obstruction, runny nose, shortness of breath, wheezing, hypoxia, and in severe cases, pneumonia and bronchiolitis [3]. Adding to that, development of asthma has been highly associated with RSV infection at early life stages [6].
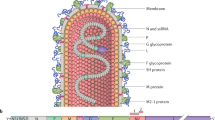
RSV genome contains more than 15,000 nucleotides coding for 11 known proteins: 2 non-structural and 9 structural proteins [7,8,9], with G, F, and SH being the surface proteins [1]. As the name signifies, attachment protein (G) helps attaching the virus to host cells, and fusion protein (F) is responsible for viral fusion and syncytium formation. Small hydrophobic (SH) protein, not-well-understood, seems to improve virus infection [10]. The matrix (M) protein serves as the inner envelope protein [11]. Four nucleocapsid-associated proteins symbolize viral transcription factors: nucleoprotein (N), phosphoprotein (P), large (L) and (M2-1) proteins [2, 12]. M2-2 regulatory protein, encoded by the downstream open reading frame of M2, is responsible for RNA synthesis during virion assembly [7]. NS1 and NS2 are non-structural proteins and are suspected to play a role in IFN-release inhibition from infected cells [13, 14] (Fig. 1).
RSV strains are classified into two subtypes: RSV-A and RSV-B. Despite sharing comparable infectivity and cross-reactive F-induced antibodies, both subtypes are antigenically distinct with about 50% genetic differences in the G gene and 10% differences in the F gene [15,16,17]. Like other respiratory viruses, RSV infections tend to increase in cold weather, low temperature, and following rainfalls. In temperate climate countries, RSV circulates throughout the winter season, but peaks between December and January [18]. Whereas in tropical countries, outbreaks of RSV occur during hot, humid, rainy days of the summer season [19].
For RSV treatment, infected young infants are mainly provided with supportive care such as oxygen supplementation and bronchial dilators. Further, Palivizumab, the only FDA-approved monoclonal antibody, is recommended as prophylaxis by the American Academy of Pediatrics (AAP) for children at high risk (e.g., immune-compromised, premature, congenital heart- or chronic lung-diseased) [20,21,22]. This antibody is limited in use for adults and elderly. Hence, vaccination is the efficient method to protect immune-compromised adults from RSV illness and complications. Still, to date, no vaccine has been licensed, specifically after the failure of different vaccine trials such as formalin-inactivated RSV vaccine that resulted in enhanced disease illness in the vaccinated group [23, 24].
G glycoprotein, a target for neutralizing antibodies, is antigenically variable making it challenging to create a broadly protective vaccine. On the other hand, F glycoprotein, another target for neutralizing antibodies, is highly conserved, yet it is present in two forms on the virion surface: one metastable structure called pre-fusion (pre-F), which is disposed to switch unpredictably to another stable post-fusion (post-F) structure [25]. Despite the fact that G and F glycoproteins provoke neutralizing antibody production, F is the major vaccine development target since it is necessary to enter host cells, and presents more epitopes targeted by neutralizing antibodies [26,27,28]. To date, six antigenic sites for neutralization have been identified on pre-F and post-F conformations. Most importantly, site Ø, a pre-F specific epitope, binds to D25, 5C4, and AM22 monoclonal antibodies which have 100-times more neutralizing activity than Palivizumab [29]. Pre-clinical experiments demonstrated the ability of a stabilized pre-F protein to induce potent antibody response in animal models and hence, it is being produced for clinical trials as a putative vaccine in pregnant women. [29,30,31] (Fig. 2).
Location of six antigenic sites on pre-fusion (left) and post-fusion (right) structure of RSV F glycoprotein and their neutralizing potency. Adopted from [3]
This review gathers data related to RSV molecular biology and pathogenesis, illustrates about immune responses to RSV infection, and discusses new vaccination approaches considering previously failed vaccine trials and promises of pre-F protein pre-clinical studies.
RSV molecular biology
RSV genome is a negative sense ssRNA containing more than 15,000 nucleotides and encodes for 11 proteins. F, G, and SH are the only glycoproteins located at virion surface, where F and G are the main targets for neutralization and vaccine development [11].
Fusion protein (F)
F protein is a class I fusion protein composed of 574 amino acids (AA). With a molecular weight of a 50 kDa C-terminal fragment F1 and a 20 kDa N-terminal fragment F2, the protein acquires a trimer of heterodimers [9]. At AA positions 109 and 136, two furin cleavages take place. This feature releases a glycopeptide and thus reveals the hydrophobic site at F1 fragment [32]. F1 and F2 are linked by a cysteine-rich region at two positions: between AA70 and AA212, and between AA37 and AA439 [9]. Other F-related features involve N-glycosylation in F1 at AA position 500, and in F2 at AA positions 27 and 70 [33]. F protein is highly conserved, with only 25 AA differences between RSV subtypes A and B [9, 11].
Attachment protein (G)
G protein is another target for neutralizing antibodies and it is a type II integral membrane protein composed of 298AA and weights ~ 90 kDa [34]. It is vastly glycosylated and it is expressed in secreted and membrane-anchored forms called Gs and Gm, respectively. Gs is linked to neutralization inhibition, while Gm is related to viral attachment [35]. This host-virus membrane attachment is mediated by heparin sulfate proteoglycans receptor interaction. The antigenic variation is situated in the mucin domain of G protein at both C- and N-terminal ends. N- and O-glycosylation enables the protein to mature and enhances immune escape mechanisms [9]. Other feature includes a central conserved region (CX3C motif) which is responsible for CX3CR1 binding to diminish inflammatory cytokines release [9,10,11, 36].
RSV pathogenesis
Studies regarding pulmonary immune cells in young infants are difficult to implement due to ethical and technical obstacles. Instead, different animal models have been used to study RSV pathogenesis, and immune responses to RSV infection [37] [35]. Further, most researchers study immune responses to RSV infection in adults more than neonatal models. For that reason, RSV pathogenesis in infants remains incompletely understood [38].
RSV is the most common agent to cause LRTI among children below the age of 5 years [19]. Most of those acquire mild to moderate disease manifestations, and about 2–3% progress to severe illness resulting in hospitalization [3]. Factors contributing to RSV pathogenesis and disease severity could be viral, host and environmental factors [39].
Viral factors
RSV has various mechanisms to enhance its pathogenesis. The main targets of this virus are type I alveolar cells and superficial airway epithelial cells [40]. RSV infection results in disruption of ciliated epithelial cells, followed by mononuclear cell infiltration, mucosal edema and section, and syncytia formation [41]. The virus also causes alternations of the cell-cycle-regulatory proteins inside the infected cells, resulting in accumulation of G0/G1 cell population and thus enhanced viral replication [42]. Infectivity, disease severity, and increased cytopathology are directly linked to viral load as well as to the strain specificity [43]. For instance, studies revealed that infection with subtype A-strain 19 causes elevated IL-13 excretion, airway hypersensitivity, and mucus production in mice [44]. A2001/2-20 isolate, on the other hand, initiates more severe complications than strain A2, strain 19, Long, and A2001/3-12, such as epithelial desquamation and bronchiolitis [45]. RSV exerts several mechanisms to trick host immunity throughout the infection process. Such mechanisms involve NS1 and NS2 proteins, which play a role in blocking the release of type I IFN (IFNa and IFNb) from infected epithelial cells via JAK/STAT pathway or toll-like receptor (TLR), causing interrupted dendritic cell recruitment and T cell activation [13]. Further, NS1 and NS2 proteins downgrade cell apoptosis through PI3k pathway activation to prolongate infected cell survival for more viral replication [46]. G glycoprotein is another source for immune escape. It is able to limit the function of CX3CL1-mediated CXCR1 leukocyte recruitment [47], decoy neutralizing antibodies, and delay antigen recognition because of its high genetic variation and glycosylation. Gs, a secreted form of G glycoprotein produced during infection, plays the role of binding to RSV antibodies in the sera, and thus, diminishing neutralization of the virus [48]. Moreover, this short form acts as a TLR antagonist to downregulate early inflammatory cytokines release from infected cells through TLR-2, TLR-4, and TLR-9 [49, 50].
Host factors
RSV-induced LRTI severity is influenced by several host factors. Generally, RSV infections are more prevalent at age extremities, among premature babies, males, those with congenital and chronic illnesses, and infants with low maternal antibody titer [39, 51]. Association between vitamin D deficiency at early life and LRTI severity has also been reported [52]. Additionally, genetic susceptibility to RSV infection has been documented. The reported genetic polymorphisms involve innate and adaptive defense genes, surfactant protein genes, host cell receptor genes, and Th1/Th2 response genes [53]. Imbalances or defects in cytokines released from infected cells as well as antigen presenting cells, influence downstream activation of adaptive immunity. Knowing the importance of cellular adaptive components in viral clearance, proper T and B cell activation and differentiation guarantee controlled RSV spread and infectivity. For that, Th1 to Th2 imbalance or Th2-biased immunity resulted in enhanced respiratory diseases following the previous vaccination trial of FI-RSV [54] [55] (Fig. 3).
Environmental factors
Exposure to smoke, besides its negative effect in general, has been reported to augment the risk of RSV infection and exacerbate RSV-induced manifestations, particularly bronchiolitis [56, 57]. Cigarette smoking directly effects primary airway epithelial cells, resulting in necrosis which induces inflammation and increases viral load [58]. Further, correlation between air pollution and severe bronchiolitis has been reported in several studies. For instance, it was shown in one study that 32% of children < 1 year who attended Paediatric Emergency Department for bronchiolitis from 2004 to 2014 in Rome had RSV infection, and disease severity in those children was significantly correlated with air pollutants levels such as benzene, nitrogen oxide and dioxide, and particulate matters. This correlation was not observed with other respiratory viruses such as in rhinovirus, suggesting that RSV pathogenesis could be environmentally enforced by air pollution [59]. Cold weather is another favorable factor for RSV spread and infection. It has been found that the virus circulates more in cold months (November to February) compared to any other time of the year [19].
Immunity to RSV
Innate immune system responses
Early-life immunity can be described in some cases as suppressive. Due to the presence of circulating maternal immunoglobulins and develo** organs in few-month-old babies, the immune system is described to be tolerant and defective in quality and quantity [38, 60]. In fact, fetal CD4+ T cells were revealed to differentiate into more T regulatory (Treg) cells as a mechanism of immune tolerance towards maternal antibodies, organ development, and microbiota colonization; making immunity adaptive to early-life needs rather than defective [61]. Nevertheless, these features make young infants more susceptible to infections compared to older children and adults. Among immature immune components, natural killer (NK) cells were found to be modulated through transforming growth factors beta (TGFβ) secreted by Treg [62]. Neutrophils as well were found to have reduced cytokines release, neutrophil extracellular traps (NETs) expression, and phagocytic function [63]. Cytokines and growth factor environment in neonatal blood is affected by higher levels of adenosine, which alters and can downregulate pro-inflammatory immune responses [64]. Neonatal dendritic cells (DCs) and subtypes, essential parties to link innate to adaptive immunity, were found to be limited in number and function as compared to adult DCs. This include the recognition, processing, and presentation of foreign antigens to T cells to induce adaptive immunity. These deficiencies tend to stimulate the immune system towards Th2 differentiation [65,66,67]. Up to the present time, immunomodulation remains an incompletely discovered process and further investigations are required to understand early-life immunity, ho** to find the appropriate methods for young infants’ protection from RSV infections.
Activation of adaptive immunity depends on the efficiency of innate immunity. Antigen presentation is the critical point to induce adaptive defenses and thus the following up functions. Viral clearance and protective antibody production are the expected results from T cell and B cell contributions. However, the early-life alternations in innate defense influence critically the whole process from adaptive immunity activation to antibody production and viral clearance [68].
Adaptive immune system responses
As mentioned above, DCs trigger T cells to activate and differentiate into T cytotoxic. This role is disturbed by every limitation occurring in DC at early life, resulting in disturbance/imbalance of immune system efficacy [69]. CD4+ T cells were found to lose balance to differentiate towards Th1 and Th2 in response to certain infectious diseases at the early stage of life, confirming that immunomodulation might result in Th2-derived immune response [70]. Previous studies have confirmed the association of enhanced disease illness resulting from FI-RSV and Th2-driven pathology [71]. CD8+ T cells play an essential role in the protection from viral infections [72]. They participate in all situations of viral clearance once the infection is striking [73]. Cases of RSV infections with decreased CD8+ levels such as in severe combined immune-deficiency (SCID), bone marrow (BM), or lung transplantation have led to death [38]. At early life, CD8+ T cells are also impaired, resulting in severe RSV infection that could be fatal [74]. Antibody production and maturation is another challenging process for the immune system at early life. The importance of antibodies manifests in some Fc-accelerated auxiliary processes of RSV clearance in young children, particularly neutralizing antibodies which reduce the number of infected cells and delay the virus from reaching the lower respiratory tract [75]. Therefore, decreased antibody production and maturation may contribute to RSV severity. This antibody inadequacy rises from B cell immaturity and limitation to differentiate into plasma cells [64]. According to one study, RSV neutralizing immunoglobulins are unlikely to develop after a natural RSV infection throughout the early few months of life. Poor humoral responses towards RSV infection could be the result of limited affinity maturation of B cells in germinal centers, which would result in limited somatic hypermutation (SHM). This could be attributed to intrinsic regulations, limitations in T cell help, or higher activation requirements of dendritic cells [64, 76]. That said, B cells start to maturate gradually at 4–6 months old, resulting in significant increase of neutralizing antibodies titer after 6 months of age [77].
These findings confirm the ineffectiveness of vaccination prior three months of age since both innate and adaptive immune systems are undeveloped and distinct from adult immunity, and instead empower the need of an alternative source of protection to compensate neonatal immunity (Fig. 4).
Early-life immunity responses towards RSV infection. Neonatal innate responses following RSV infection are weak: low levels of cytokines, e.g., interferons, diminished TLRs signals, altered antigen-presenting function, and reduced Treg activation. These observations result in a skewed adaptive immune response towards Th2 and low CTL activation through Th1. Weakened Tfh activation and B cell memory production and antibody production inhibition by IFN-γ result in low titer and low-affinity antibodies. Consequences of suppressive immune response lead to bronchiolitis in susceptible infants (adopted from [70])
RSV mechanisms to escape host immunity
The ability of RSV to reinfect individuals throughout life is attributed partially to the weakening of host immunity to this pathogen over time. Further, the virus evolved various mechanisms to avoid the immune system despite its limited genetic variation. The virus exerts three main classes of immune escape: (1) location-related infectivity, (2) conformational avoidance from neutralization/neutralizing antibody, and (3) functional change of the immune responses. Infecting the ciliated epithelial cells (superficial) of the respiratory airways saves or delays the virus from getting exposed to dendritic cells that are responsible for transporting RSV antigen to lymph node to recruit immune reactions [40, 78]. To avoid neutralizing antibodies, the structure of F protein, responsible for virus-host and cell-cell fusion, changes conformation from a metastable to stable form so-called pre-F and post-F, respectively. Since pre-F is the required part for viral fusion and it is the target for more potent neutralizing antibodies, RSV balances between both functions by transitioning to post-F in a smartly calculated manner to enter the cell (remain infectious) and escape neutralization by pre-F-specific antibodies [79, 80]. Lastly, modulating host immunity initiates when RSV targets young infants. Because young infants have naive immune system (immature dendritic cells, deficiency of B cell somatic hypermutation) [70], the consequences of an RSV infection might highly result in a Th2-favorized immune responses. Besides, the virus is able to (1) inhibit type I IFN release from infected epithelial cells through NS1 and NS2 proteins [13], (2) manipulate dendritic cell signaling through G glycoprotein [81], and (3) disguise antibody neutralization through the secreted form of G glycoprotein [48].
RSV vaccine history and enhanced respiratory illness
Although RSV disease is believed to be an ancient agent, the virus was first identified in 1955 as “Chimpanzee Coryza Agent,” then a year later was linked with bronchiolitis in young children [82]. A few years later, a vaccine attempt was done using formalin-inactivated RSV given to young children. The idea of producing a formalin-inactivated RSV vaccine was inspired by previous successful trials on other viruses such as poliovirus [83]. In four separate studies, the FI-RSV vaccine was given at a 3-dose regimen (0, 1, 4 months) for 2–7 month-old infants [80]. Unfortunately, the experiment failed and resulted in enhanced respiratory diseases following a natural RSV infection, in which 16/20 (80%) of the vaccinated children were hospitalized and 2/20 (10%) passed away [83]. Vaccine failure was attributed to several immunological features associated with the humoral and cellular responses. Induction of binding antibodies with low neutralizing activity [84] resulted in the formation of immune complexes deposition and complement activation in airways [38, 80]. Eosinophilia was another characteristic of FI-RSV vaccination, causing hypersensitive peri-bronchiolar inflammation regulated by IL-4, IL-5, IL-13, and IgE [54]. In addition, following RSV infection in FI-RSV vaccinated individuals, triggered Th2-driven immunity and low CD8+ T cells activation was observed [85].
Recent studies demonstrated that vaccination with pre-F protein elicits stronger neutralizing antibodies as compared to post-F. Yet, based on one study, the expression of pre-F antigens on the surface of the virus was almost lost within the 72 h of formalin treatment [80]. Consequently, to develop an effective vaccine without complications such as enhanced illnesses, it is critical to consider the consequences of viral inactivation and formalin concentration [86, 87]. Viral inactivation alters antigenicity and immunogenicity. Formalin, a chemical fixative/preservative to inactivate viruses, creates different outcomes using different concentrations, where a high concentration of 1% destroys viral infectivity via inter- and intra-protein cross-linkage, and lower concentrations preserve antigenic epitopes differently [88, 89]. Following FI-RSV vaccine experimentation, other trials using live-attenuated virus vaccine were conducted. Side effects were also observed with such vaccines due to inaccurate degree of attenuation, and therefore, were not approved for failing to preserve antigenicity and immunogenicity [38, 71].
F protein-based neutralization
Structures of pre-F, post-F proteins and their corresponding epitopes are depicted in Fig. 2. Several recent studies have demonstrated the relationship between pre-F and post-F binding antibodies and their neutralizing activities. Structural and immunological analysis demonstrated that pre-F-specific antibodies are at least 8-fold more potent than shared (pre-F and post-F cross-reactive) antibodies and 80-fold more potent than post-F-specific antibodies [31, 86]. Ngwuta et al. showed in a 2015 publication that adsorption of sera from individuals aged between 7 and 93 years with stabilized pre-F protein removed > 90% of neutralizing activity and diminished binding antibodies to both F conformations. On the other hand, sera adsorbed with post-F retained most of the neutralizing activity (> 70%) as well as the binding antibodies to pre-F conformation [27]. These findings demonstrate a positive correlation between pre-F binding and virus neutralization [90], while the correspondence of post-F binding and neutralization is not significant [9]. The study further investigated neutralization activity at the epitope level to compare well-identified antigenic sites present on pre-F and post-F conformations. Sixty-percent of highly potent neutralizing antibodies were directed to sites Ø and V, which are only present on the pre-F conformation. Meanwhile, sites III and IV, shared between pre-F and post-F, represented targets for moderate neutralizing antibodies, followed by sites I and II, which represented targets for weak neutralizing antibodies [4]. These differences in neutralization towards both F conformations could possibly be explained by the uniqueness, the accessibility, and the approach angle of each antigenic site [31].
Palivizumab and recently discovered RSV antibodies
Palivizumab is the only licensed humanized monoclonal antibody and is designed to identify a shared antigenic epitope between pre-F and post-F, site II, in the hope of increasing its target spectrum. Determination of pre-F structure in complex with D25 antibody [29] and subsequent stabilization of the protein with cavity-filling hydrophobic substitution mutations [86] enabled a better understanding of the immune response to the two forms of this metastable protein. It was found that pre-F preserves more antigenic sites compared to post-F, which are the target for more potent neutralizing antibodies than Palivizumab’s antigenic site. Various studies have compared the neutralizing potency of site II to particularly pre-F-specific epitopes. Palivizumab or 1129 antibody showed comparable binding affinity to its appropriate antigenic site on both forms of the protein. However, site Ø-specific antibody 5C4 exhibited 50-fold more neutralizing activity than 1129 in mice and decreased RSV titers by 1000-fold more than Palivizumab, suggesting that site Ø elicits more protective antibodies from RSV infection than site II [90]. Site V, another pre-F-specific site, was also investigated against Palivizumab-targeted site II. AM14, a quaternary pre-F-specific antibody, neutralized all tested RSV strains, with IC50s of 13.6 ng/ml for strain A Long, 12.4 ng/ml for strain A2, 30.8 ng/ml for subtype B strain 18,537, and 4.6 ng/ml for subtype B strain 9320, compared to Palivizumab which neutralized the same strains with IC50s of 300, 320, 380, and 120 ng/ml, respectively [91]. These data suggested that antibodies to site V are 100-fold more potent than site II directed antibodies. Recently, a new pre-F-specific epitope was discovered and is referred to as site VIII. This site was shown to reside between site II and site Ø in the pre-f conformation. mAb 90, specific to this new site, neutralized three tested RSV strains of A2, 18,537 B, and Long with IC50s of 4, 10, and 35 ng/ml, respectively, compared to Palivizumab neutralization activity of 1900, 1300, and 212 ng/ml—IC50 of the same RSV strains [25]. These findings as well confirm 1000-fold more potency of site VIII than site II. The main conclusion from this comparison is to emphasize on the important role of pre-F-specific antibodies in RSV infection prevention and the potential use of pre-F antigen as an effective vaccine. It is worth noting that Palivizumab antibody is very costly, given only to high-risk premature infants, restricted for adults with immunodeficiency or elderly people, and has low in neutralizing activity. The discovery of new highly potent antibodies that target pre-F protein will promote better RSV treatment and care management.
RSV vaccine approaches
The reverse outcomes of FI-RSV vaccine and virus-attenuated trials invigorated researchers to develop and test new vaccines concepts that avoid side effects and complications. The nature of RSV infectivity, the host immune responses at early life including the impact of imbalanced Th1 to Th2 immunopathology, and the viral mechanisms to escape immunity, are all decisive points for develo** RSV vaccine. There are four groups that are considered the main targets for RSV vaccination including young infants, pregnant females, immunocompromised adults, and elderly people. It is acknowledged that infection with RSV is more dramatic in infants less than 3 months of age. Implementing an efficient primary vaccination at this stage has been challenging considering the immature neonatal immunity, the presence and the waning of maternally derived antibodies, and the actual impact of natural primary RSV infection. Accordingly, various vaccine platforms and approaches are being considered to protect this vulnerable group [92]. Inactivated and subunit RSV vaccines pose significant safety concerns as they have been associated with enhanced RSV disease in RSV-naive infants. On the other hand, live-attenuated RSV strains have been rarely associated with enhanced RSV disease. Just recently, a live-attenuated RSV vaccine candidate, containing stabilized temperature-sensitivity mutations, was tested in infants between 6 and 24 months of age. This reverse genetics-generated vaccine (RSVcps2) was shown to be well tolerated and moderately immunogenic and had increased genetic stability in 6–24-month-old RSV-seronegative children [93]. The safety and efficacy of this vaccine in infants under 3 months of age is still to be evaluated. Considering safety issue of vaccines in this age group, alternative sources of protection are being considered to support young infants during their first months of life. Passive immunity can reduce RSV burden if enough protective maternal antibody titers are delivered to the neonates [70]. Vaccinating pregnant women few months prior delivery to boost pre-existing pre-F-specific antibodies and enhance maternal antibody transfer to infants is being considered as an alternative vaccination approach [3]. Transferred maternal antibodies would protect young infants for up to 6 months, which is enough for the immune system to maturate and develop anti-RSV antibodies in the successive RSV infections [70]. This potential vaccine could be later administrated to children between 6 and 12 months old once their immune system is completely developed. On the other hand, immunocompromised adults and elderly people could benefit as well from vaccination programs to avoid RSV-complications at this stage of life where disease prevalence is high and immunity is weakened.
To meet the needs and characteristics of each population, selection of appropriate adjuvant plays a crucial role in vaccine immunogenicity and effictivness. Adjuvants are molecules coupled with the vaccines to serve as a transporter or/and immune inducer particularly in immunocompromised individuals [94]. One recent study assessed the impact of different adjuvants on vaccine efficiency and immune responses in animal models. It was found that an oil-in-water adjuvant (Sigma adjuvant system (SAS) plus Carbopol) induced the highest RSV neutralizing antibody responses, followed by Alum, SAS alone, AdjuPlex, and Poly (I:C). TLR4 agonist MPLA, Alum plus MPLA, or AddaVax generated moderate responses. All these findings were compared to DS-Cav1 (stabilized pre-F protein) alone without adjuvant which induced a much inferior response. When elderly mice with pre-existing immunity against DS-Cav1 were tested for an immune boost with DC-Cav1 plus an adjuvant, results showed that SAS plus Carbopol enhanced immune response 2- to 3-folds, while Alum enhanced it by 5-folds [95]. Similarly, human immune responses to pre-F vaccination would differ according to the adjuvants used in the vaccine formulation. For instance, AS04 and MF59 adjuvants enhance viral vaccine immunogenicity in human compared to vaccines without adjuvants [94]. In a study, first-in-human inspecting immune responses to RSV F vaccine, the investigators introduced the vaccine randomly to elderly subjects (> 60 years old) with or without TLR-4 agonist (glucopyranosyl lipid A), and stable emulsion adjuvant (GLA-SE). Within 1 week of vaccination, 50% of patients showed 3-fold more neutralizing antibody titers than placebo subjects at the highest vaccine dose, and by day 29, all patients exhibited similar responses. Further, functional T cells were detected after 8 days post-vaccination with pre-F protein as measured by IFN-γ cytokine release using ELISPOT stimulation assay. At the highest dose, 74% of vaccines with pre-F+GLA-SE expressed 3-fold more IFN-γ above control. Overall, this phase I study demonstrated a dose-dependent human immune responses to stable RSV F protein among elderly subjects, where adjuvant (GLA-SE) use augmented humoral and cellular immunity significantly compared to placebo group [96].
The use of Palivizumab highlights the importance of neutralizing antibodies in protection from severe RSV infection. Further characterization of pre-F specific antibodies, particularly those to site Ø that are 100- to 1000-fold more potent than the licensed monoclonal antibody, has opened the door towards using pre-F as a putative vaccine [3, 25, 27, 90]. Other strategies and delivery methods involve the use of vectors [97] and nucleic acids [98]. Liu X. et al. have recently claimed a pre-clinical development of RSV F vaccine by expressing a stabilized pre-F via human parainfluenza type 1 (HPIV1) recombinant vector. RSV F was expressed as a full-length protein or as a chimeric form with HPIV1 F transmembrane and cytoplasmic tail (TMCT) domains. Results showed that full-length was more immunogenic in terms of neutralization and protection following RSV challenge, compared to the chimeric form with HPIV1 F TMCT modification, which had reduced immunogenicity [99]. Another study by Liang et al. used human parainfluenza type 3 as a vector to deliver the vaccine and codon-optimized pre-F gene was incorporated. Optimized-codon with low CpG enabled the virus to better express pre-F protein, reduce IFN release, and replicate efficaciously in vivo. This bivalent RSV/HPIV3 vaccine, combining stable pre-F (DS-Cav1), low CpG content, and a vector package, improved F immunogenicity, induced higher complement-independent antibodies titers and resulted in better protection following RSV challenge in hamsters [100].
All these vaccination approaches utilize a stabilized and conserved pre-F for the induction of highly potent and protective antibody responses. The antigen-naïve population is the main target for these vaccine trials, yet, older children, immunocompromised adults, and elderly people are potential candidates for RSV vaccination.
Summary
RSV remains an important viral agent for bronchiolitis in infants despite the availability of supportive and prophylactic treatments. As long as develo** an effective vaccine to limit RSV burden is still a challenge, the menace continues to hunt individuals at high risks including neonates. The ability of this virus to manipulate the immune system in general and suppress neonatal immune responses in particular through G glycosylation and F conformational alternation, is the main element of RSV pathogenesis. Following the several failed vaccine trials since 1960s, researchers are now focusing their investigations on F glycoprotein as a leading candidate for vaccination, considering its conservation and presentation of multiple neutralizing epitopes. In fact, structure-based stabilization of pre-F protein through cavity-filling mutations enabled better understanding of the immune response to this protein, including the discovery of highly potent antibodies that target pre-F-specific antigenic sites. The ultimate goal is to utilize stabilized-pre-F molecule as an effective vaccine, considering the high expense of antibody therapy, which is also restricted in use. Neonates are partially deficient in inducing adequate antibody responses before three months of age. Nonetheless, passive immunity through maternal antibodies would protect newborns during the first few months of life. Induction of high maternal antibody response via vaccination would rely on the use of appropriate vaccine formulation, including adjuvants, which are highly debatable for their side effects. Presence and level of pre-existing immunity are additional factors to consider while develo** vaccination strategies. Effects of adjuvants and pre-existing immunity could be evaluated in clinical trials with adult populations. In fact, few groups have recently lunched phase I to III clinical trials to evaluate various RSV vaccine platforms and adjuvant formulations. So far, adjuvanted-pre-F vaccination is showing promising results in the elderly population [96]. Further, a study examining the immunogenicity and safety of pre-F nanoparticle-plus Alum adjuvant vaccine in healthy pregnant women is currently running in the USA by Novavax company. The study, which is anticipated to be completed in May 2020, aims to determine the safety and efficacy of this vaccine in protecting young infants from RSV infection after 90 days from delivery through maternal immunization [101].
References
Rey-Jurado E, Kalergis AM (2017) Immunological features of respiratory syncytial virus-caused pneumonia—implications for vaccine design. Int J Mol Sci 18(3):556
Nair H et al (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375(9725):1545–1555
Graham BS (2017) Vaccine development for respiratory syncytial virus. Curr Opin Virol 23:107–112
Widjaja I et al (2016) Characterization of epitope-specific anti-respiratory syncytial virus (anti-RSV) antibody responses after natural infection and after vaccination with formalin-inactivated RSV. J Virol 90(13):5965–5977
Varga SM, Braciale TJ (2013) The adaptive immune response to respiratory syncytial virus. Curr Top Microbiol Immunol 372:155–171
Sawadkohi RB et al (2012) Prevalence of acute lower respiratory tract infections due to respiratory syncytial virus in Amirkola Children’s Hospital, Northern Iran during March 2008–March 2010. Iran Red Crescent Med J 14(10):680
Collins PL (1991) The molecular biology of human respiratory syncytial virus (RSV) of the genus pneumovirus. In: Kingsbury DW (eds) The paramyxoviruses. The viruses. Springer, Boston
Collins PL et al (1986) Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci 83(13):4594–4598
Graham BS, Modjarrad K, McLellan JS (2015) Novel antigens for RSV vaccines. Curr Opin Immunol 35:30–38
Tripp RA, Jorquera PA (2016) Human respiratory syncytial virus. Springer, Berlin
Kingsbury DW (2012) The paramyxoviruses. Springer Science & Business Media, Berlin
Li Y et al (2014) Inhibition of the human respiratory syncytial virus small hydrophobic protein and structural variations in a bicelle environment. J Virol 88(20):11899–11914
Barik S (2013) Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr Top Microbiol Immunol 372:173–191
Chin V et al (2016) Design and validation of small interfering RNA on respiratory syncytial virus M2-2 gene: a potential approach in RNA interference on viral replication. J Virol Methods 236:117–125
Chamat S et al (1999) Human monoclonal antibodies isolated from spontaneous Epstein-Barr virus—transformed tumors of Hu-SPL-SCID mice and specific for fusion protein display broad neutralizing activity toward respiratory syncytial virus. J Infect Dis 180(2):268–277
Fuentes S et al (2016) Antigenic fingerprinting following primary RSV infection in young children identifies novel antigenic sites and reveals unlinked evolution of human antibody repertoires to fusion and attachment glycoproteins. PLoS Pathog 12(4):e1005554
Mufson MA et al (1985) Two distinct subtypes of human respiratory syncytial virus. J Gen Virol 66(10):2111–2124
Aamir UB et al (2013) Molecular characterization of circulating respiratory syncytial virus (RSV) genotypes in gilgit baltistan province of Pakistan during 2011-2012 winter season. PLoS One 8(9):e74018
Al-Toum R, Bdour S, Ayyash H (2006) Epidemiology and clinical characteristics of respiratory syncytial virus infections in Jordan. J Trop Pediatr 52(4):282–287
Turner TL et al (2014) Respiratory syncytial virus: current and emerging treatment options. Clinicoecon Outcomes Res 6:217
Diseases CoI (2009) Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 124(6):1694–1701
Simoes EA (1999) Respiratory syncytial virus infection. Lancet 354(9181):847–852
Modjarrad K et al (2016) WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization meeting held on 23–24 March 2015. Vaccine 34(2):190–197
Simões EA et al (2015) Challenges and opportunities in develo** respiratory syncytial virus therapeutics. J Infect Dis 211(suppl_1):S1–S20
Mousa JJ et al (2017) A novel pre-fusion conformation-specific neutralizing epitope on the respiratory syncytial virus fusion protein. Nat Microbiol 2:16271
Magro M et al (2012) Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci 109(8):3089–3094
Ngwuta JO et al (2015) Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7(309):309ra162–309ra162
Beeler JA, van Wyke Coelingh K (1989) Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol 63(7):2941–2950
McLellan JS et al (2013) Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340(6136):1113–1117
Krarup A et al (2015) A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun 6:8143
Gilman MS et al (2016) Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol 1(6). https://doi.org/10.1126/sciimmunol.aaj1879
Zhao X et al (2000) Structural characterization of the human respiratory syncytial virus fusion protein core. Proc Natl Acad Sci 97(26):14172–14177
Tian D et al (2017) Structural basis of respiratory syncytial virus subtype-dependent neutralization by an antibody targeting the fusion glycoprotein. Nat Commun 8(1):1877
Graham BS (2016) Vaccines against respiratory syncytial virus: the time has finally come. Vaccine 34(30):3535–3541
Guzman E, Taylor G (2015) Immunology of bovine respiratory syncytial virus in calves. Mol Immunol 66(1):48–56
Wertz GW et al (1985) Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci 82(12):4075–4079
Openshaw PJ (2013) The mouse model of respiratory syncytial virus disease. Curr Top Microbiol Immunol 372:359–369
Ruckwardt TJ, Morabito KM, Graham BS (2016) Determinants of early life immune responses to RSV infection. Curr Opin Virol 16:151–157
Watkiss ER (2012) Pathogenesis of respiratory syncytial virus. Curr Opin Virol 2(3):300–305
Johnson JE et al (2007) The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 20(1):108–119
Wright PF et al (2005) Growth of respiratory syncytial virus in primary epithelial cells from the human respiratory tract. J Virol 79(13):8651–8654
Wu W et al (2011) Characterization of the interaction between human respiratory syncytial virus and the cell cycle in continuous cell culture and primary human airway epithelial cells. J Virol 85(19):10300–10309
DeVincenzo JP et al (2010) Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182(10):1305–1314
Moore ML et al (2009) A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol 83(9):4185–4194
Stokes KL et al (2011) Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 85(12):5782–5793
Bitko V et al (2007) Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-κB-dependent, interferon-independent mechanism and facilitate virus growth. J Virol 81(4):1786–1795
Tripp RA et al (2001) CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2(8):732–738
Bukreyev A et al (2008) The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol 82(24):12191–12204
Polack FP et al (2005) The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci U S A 102(25):8996–9001
Lukacs NW et al (2008) Regulation of immunity to respiratory syncytial virus by dendritic cells, toll-like receptors, and notch. Viral Immunol 21(2):115–122
Collins PL, Graham BS (2008) Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82(5):2040–2055
Belderbos ME et al (2011) Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 127(6):e1513–e1520
Miyairi I, DeVincenzo JP (2008) Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev 21(4):686–703
Knudson CJ et al (2015) RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog 11(3):e1004757
Littel-van den Hurk SV et al (2007) Immunopathology of RSV infection: prospects for develo** vaccines without this complication. Rev Med Virol 17(1):5–34
Karr CJ et al (2009) Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res 109(3):321–327
Bradley JP et al (2005) Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics 115(1):e7–e14
Groskreutz DJ et al (2009) Cigarette smoke alters respiratory syncytial virus–induced apoptosis and replication. Am J Respir Cell Mol Biol 41(2):189–198
Evangelisti M et al (2015) Air pollution and bronchiolitis from 2004 to 2014 in Rome. Eur Respir J 46. https://doi.org/10.1183/13993003.congress-2015.PA4505
Gervassi AL, Horton H (2014) Is infant immunity actively suppressed or immature? Virology 5:1
Mold JE et al (2010) Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 330(6011):1695–1699
Ndure J, Flanagan KL (2014) Targeting regulatory T cells to improve vaccine immunogenicity in early life. Front Microbiol 5:477
Dowling DJ, Levy O (2014) Ontogeny of early life immunity. Trends Immunol 35(7):299–310
Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10(9):1171–1184
Cormier SA et al (2014) Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol 88(16):9350–9360
Ruckwardt TJ et al (2014) Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog 10(2):e1003934
Marr N et al (2014) Attenuation of respiratory syncytial virus–induced and RIG-I–dependent type I IFN responses in human neonates and very young children. J Immunol 192(3):948–957
Malloy AM, Falsey AR, Ruckwardt TJ (2013) Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr Top Microbiol Immunol 372:211–231
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16(4):343–353
Lambert L et al (2014) Immunity to RSV in early-life. Front Immunol 5:466
Karron RA, Buchholz UJ, Collins PL (2013) Live-attenuated respiratory syncytial virus vaccines. In: Anderson L, Graham B (eds) Challenges and opportunities for respiratory syncytial virus vaccines. Current Topics in Microbiology and Immunology, vol 372. Springer, Berlin, Heidelberg
Liu J et al (2016) A numerically subdominant CD8 T cell response to matrix protein of respiratory syncytial virus controls infection with limited immunopathology. PLoS Pathog 12(3):e1005486
Morabito KM et al (2017) Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol 10(2):545–554
Welliver TP et al (2007) Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 195(8):1126–1136
Groothuis JR et al (1995) Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Pediatrics 95(4):463–467
R., S.C.a.A (2009) B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 9(3):185–194
Sande CJ, Cane PA, Nokes DJ (2014) The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine 32(37):4726–4729
Zhang L et al (2002) Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76(11):5654–5666
Hambling M (1964) Survival of the respiratory syncytial virus during storage under various conditions. Br J Exp Pathol 45(6):647
Killikelly AM, Kanekiyo M, Graham BS (2016) Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci Rep 6:34108
Johnson TR, McLellan JS, Graham BS (2012) Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J Virol 86(3):1339–1347
Morris J, Blount R Jr, Savage R (1956) Recovery of cytopathogenic agent from chimpanzees with goryza. Proc Soc Exp Biol Med 92(3):544–549
KIM HW et al (1969) Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine 1 2. Am J Epidemiol 89(4):422–434
Murphy BR, Walsh EE (1988) Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol 26(8):1595–1597
Polack FP (2015) The changing landscape of respiratory syncytial virus. Vaccine 33(47):6473–6478
McLellan JS et al (2013) Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342(6158):592–598
Moghaddam A et al (2006) A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med 12(8):905–907
Clemens R et al (1995) Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis 171(Supplement_1):S44–S49
Furesz J, Scheifele DW, Palkonyay L (1995) Safety and effectiveness of the new inactivated hepatitis A virus vaccine. CMAJ 152(3):343
Zhao M et al (2017) Discovery of a prefusion RSV F-specific monoclonal antibody that provides greater in vivo protection than the murine precursor of Palivizumab. J Virol 91(15). https://doi.org/10.1128/JVI.00176-17
Gilman MS et al (2015) Characterization of a prefusion-specific antibody that recognizes a quaternary, cleavage-dependent epitope on the RSV fusion glycoprotein. PLoS Pathog 11(7):e1005035
Anderson L et al (2013) Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 31:B209–B215
Buchholz UJ et al (2018) Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis 217(9):1338–1346
Shah RR, Hassett KJ, Brito LA (2017) Overview of vaccine adjuvants: introduction, history, and current status. Methods Mol Biol 1494:1–13
Sastry M et al (2017) Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One 12(10):e0186854
Falloon J et al (2016) A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 34(25):2847–2854
Taylor G et al (2015) Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci Transl Med 7(300):300ra127–300ra127
Pardi N, Weissman D (2017) Nucleoside modified mRNA vaccines for infectious diseases. Methods Mol Biol 1499:109–121
Liu X et al (2017) Attenuated human parainfluenza virus type 1 expressing the respiratory syncytial virus (RSV) fusion (F) glycoprotein from an added gene: effects of prefusion stabilization and packaging of RSV F. J Virol 91(22):e01101-17
Liang B et al (2017) Improved prefusion stability, optimized codon-usage, and augmented virion packaging enhance the immunogenicity of respiratory syncytial virus (RSV) fusion protein in a vectored vaccine candidate. J Virol 91(15). https://doi.org/10.1128/JVI.00189-17
Novavax. A study to determine the safety and efficacy of the RSV F vaccine to protect infants via maternal immunization. @ NIH clinical trial: https://clinicaltrials.gov/ct2/show/NCT02624947
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
N/A
Informed consent
N/A
Rights and permissions
About this article
Cite this article
Taleb, S.A., Al Thani, A.A., Al Ansari, K. et al. Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches. Eur J Clin Microbiol Infect Dis 37, 1817–1827 (2018). https://doi.org/10.1007/s10096-018-3289-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3289-4