Abstract
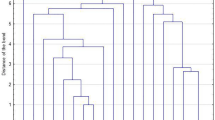
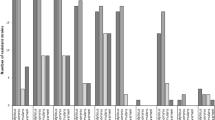
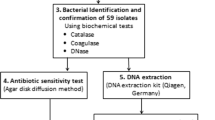
Staphylococcus lugdunensis has emerged as a significant human pathogen, with distinct clinical and microbiological characteristics. Our goal was to identify the virulence factors in S. lugdunensis recovered from infected patients of two Greek hospitals during a six-year period (2008–2013). A collection of 38 S. lugdunensis was tested for biofilm formation, antimicrobial susceptibility, clonal distribution, virulence factors (ica operon, fbl, atlL, vwbl, slush) and antibiotic resistance genes (mecA, ermC) carriage. Strains were classified into pulsotypes by pulsed-field gel electrophoresis (PFGE) of SmaI DNA digests. The majority (22) was isolated from skin and soft tissue infections (SSTIs), nine from deep-sited infections (DSIs), including three bacteraemias and seven from prosthetic device-associated infections (PDAIs). All isolates were oxacillin-susceptible, mecA-negative and fbl-positive. The highest resistance rate was detected for ampicillin (50 %), followed by erythromycin and clindamycin (18.4 %). Fourteen isolates (36.8 %) produced biofilm, whereas 26/38 (68.4 %) carried the ica operon. Biofilm formation was more frequent in isolates from PDAIs. Thirty-six strains (94.7 %) carried atlL and 31 (81.6 %) carried vwbl, whereas slush was detected in 15 (39.5 %). PFGE revealed a low level of genetic diversity: strains were classified into seven pulsotypes, with two major clones (C: 22 and D: nine strains). Type C strains recovered from all infection sites prevailed in biofilm formation and ermC carriage, whereas type D strains associated with SSTIs and DSIs carried more frequently vwbl, slush or both genes. Despite susceptibility to antimicrobials, the clonal expansion and carriage of virulence factors, combined with biofilm-producing ability, render this species an important pathogen that should not be ignored.

Similar content being viewed by others
References
Frank KL, Del Pozo JL, Patel R (2008) From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21(1):111–133. doi:10.1128/CMR.00036-07
Frank KL, Reichert EJ, Piper KE, Patel R (2007) In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother 51(3):888–895. doi:10.1128/AAC.01052-06
van der Mee-Marquet N, Achard A, Mereghetti L, Danton A, Minier M, Quentin R (2003) Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J Clin Microbiol 41(4):1404–1409
Liu C, Shen D, Guo J, Wang K, Wang H, Yan Z, Chen R, Ye L (2012) Clinical and microbiological characterization of Staphylococcus lugdunensis isolates obtained from clinical specimens in a hospital in China. BMC Microbiol 12:168. doi:10.1186/1471-2180-12-168
Spiliopoulou I, Petinaki E, Papandreou P, Dimitracopoulos G (2004) erm(C) is the predominant genetic determinant for the expression of resistance to macrolides among methicillin-resistant Staphylococcus aureus clinical isolates in Greece. J Antimicrob Chemother 53(5):814–817. doi:10.1093/jac/dkh197
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193
Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R (1996) The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178(1):175–183
Spiliopoulou AI, Krevvata MI, Kolonitsiou F, Harris LG, Wilkinson TS, Davies AP, Dimitracopoulos GO, Karamanos NK, Mack D, Anastassiou ED (2012) An extracellular Staphylococcus epidermidis polysaccharide: relation to Polysaccharide Intercellular Adhesin and its implication in phagocytosis. BMC Microbiol 12:76. doi:10.1186/1471-2180-12-76
Nilsson M, Bjerketorp J, Wiebensjö A, Ljungh A, Frykberg L, Guss B (2004) A von Willebrand factor-binding protein from Staphylococcus lugdunensis. FEMS Microbiol Lett 234(1):155–161. doi:10.1016/j.femsle.2004.03.024
Vischer UM, de Moerloose P (1999) von Willebrand factor: from cell biology to the clinical management of von Willebrand’s disease. Crit Rev Oncol Hematol 30(2):93–109
Mitchell J, Tristan A, Foster TJ (2004) Characterization of the fibrinogen-binding surface protein Fbl of Staphylococcus lugdunensis. Microbiology 150(Pt 11):3831–3841. doi:10.1099/mic.0.27337-0
Chatzigeorgiou KS, Siafakas N, Petinaki E, Zerva L (2010) fbl gene as a species-specific target for Staphylococcus lugdunensis identification. J Clin Lab Anal 24(2):119–122. doi:10.1002/jcla.20352
Donvito B, Etienne J, Greenland T, Mouren C, Delorme V, Vandenesch F (1997) Distribution of the synergistic haemolysin genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol Lett 151(2):139–144
Gibert L, Didi J, Marlinghaus L, Lesouhaitier O, Legris S, Szabados F, Pons JL, Pestel-Caron M (2014) The major autolysin of Staphylococcus lugdunensis, AtlL, is involved in cell separation, stress-induced autolysis and contributes to bacterial pathogenesis. FEMS Microbiol Lett 352(1):78–86. doi:10.1111/1574-6968.12374
The European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0, 2014. http://www.eucast.org
Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8):891–899. doi:10.1111/j.1600-0463.2007.apm_630.x
Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S (1991) Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29(10):2240–2244
Sandoe JA, Longshaw CM (2001) Ventriculoperitoneal shunt infection caused by Staphylococcus lugdunensis. Clin Microbiol Infect 7(7):385–387
Szabados F, Nowotny Y, Marlinghaus L, Korte M, Neumann S, Kaase M, Gatermann SG (2011) Occurrence of genes of putative fibrinogen binding proteins and hemolysins, as well as of their phenotypic correlates in isolates of S. lugdunensis of different origins. BMC Res Notes 4:113. doi:10.1186/1756-0500-4-113
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain ty**. J Clin Microbiol 33(9):2233–2239
Miragaia M, Carriço JA, Thomas JC, Couto I, Enright MC, de Lencastre H (2008) Comparison of molecular ty** methods for characterization of Staphylococcus epidermidis: proposal for clone definition. J Clin Microbiol 46(1):118–129. doi:10.1128/JCM.01685-07
Santos Sanches I, Mato R, de Lencastre H, Tomasz A; CEM/NET Collaborators and the International Collaborators (2000) Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb Drug Resist 6(3):199–211
Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, Mueller NJ (2008) Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 36(4):314–321. doi:10.1007/s15010-008-7287-9
Lambe DW Jr, Jeffery C, Ferguson KP, Cooper MD (1994) Examination of the glycocalyx of four species of Staphylococcus by transmission electron microscopy and image analysis. Microbios 78(316):133–143
Frank KL, Patel R (2007) Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect Immun 75(10):4728–4742. doi:10.1128/IAI.00640-07
Pereira EM, Teixeira CA, Alvarenga AL, Schuenck RP, Giambiagi-Demarval M, Holandino C, Mattos-Guaraldi AL, dos Santos KR (2012) A Brazilian lineage of Staphylococcus lugdunensis presenting rough colony morphology may adhere to and invade lung epithelial cells. J Med Microbiol 61(Pt 4):463–469. doi:10.1099/jmm.0.033001-0
Hellbacher C, Törnqvist E, Söderquist B (2006) Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin Microbiol Infect 12(1):43–49. doi:10.1111/j.1469-0691.2005.01296.x
Bourgeois I, Camiade E, Biswas R, Courtin P, Gibert L, Götz F, Chapot-Chartier MP, Pons JL, Pestel-Caron M (2009) Characterization of AtlL, a bifunctional autolysin of Staphylococcus lugdunensis with N-acetylglucosaminidase and N-acetylmuramoyl-l-alanine amidase activities. FEMS Microbiol Lett 290(1):105–113. doi:10.1111/j.1574-6968.2008.01414.x
Acknowledgements
We thank Anastasia Spiliopoulou MD, PhD for her assistance in collecting the isolates. The Ethics Committee of the University Hospital of Patras approved this study and waived the need for informed consent (approval no.: 316). Part of this work was presented as a poster presentation at the 24th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2014), May 2014, Barcelona, Spain.
Funding
This research was supported by funding from the National Staphylococcal Reference Laboratory, Greece, under the scientific responsibility of I.S. and E.D.A. (grant C954, Hellenic Centre for Disease Control and Prevention, HCDCP/KEELPNO).
Conflict of interest
Nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giormezis, N., Kolonitsiou, F., Makri, A. et al. Virulence factors among Staphylococcus lugdunensis are associated with infection sites and clonal spread. Eur J Clin Microbiol Infect Dis 34, 773–778 (2015). https://doi.org/10.1007/s10096-014-2291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2291-8