Abstract
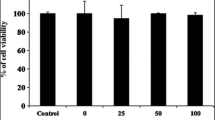
In our previous work, Asterina pectinifera was fermented with Cordyceps militaris mycelia to improve its bioactivities and was reported to have strong antioxidant activities. The aim of the current study was to investigate its anti-inflammatory effect and mechanisms of action. In this study, we observed the inhibitory effect of the extract from fermented A. pectinifera with C. militaris mycelia (FACM) on nitric oxide (NO) production and its molecular mechanism in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. FACM could decrease LPS-induced NO production. Western blot analysis showed that FACM could down-regulate LPS-induced expression of inducible NO synthase without affecting cyclooxygenase-2. Moreover, FACM exhibited anti-inflammatory activity in LPS-induced RAW264.7 mouse macrophage cells through proinflammatory mediators including TNF-α and IL-6 via nuclear factor kappa B pathway. FACM inhibited LPS-induced phosphorylation of extracellular-signal-regulated kinase expression. Our results suggest that FACM may be a potential candidate for inflammation therapy by attenuating the generation of cytokines, production of NO, and generation of ROS in RAW264.7 cells.






Similar content being viewed by others
References
Kang KH, Kim JM. The predation of trumpet shell, Charonia sp., on eight different marine invertebrate species. Aquacult Res. 35: 1202–1206 (2004).
National Federat. Fish. Cooperate, Report of National Federation of Fisheries Cooperatives about starfish removal project, Seoul (2011).
Seo JK. Conformation and biological activity of Mastoparan B and its analogs: Isolation and characterization of the biological substances from inshore hagfish (Eptatretus burgeri) skin and starfish (Asterina pectinifera), Thesis, Pukyong National University (1997).
Jo WS, Choi YJ, Kim HJ, Nam BH, Lee GA, Seo SY, Lee SW, Jeong MH. Methanolic extract of Asterina pectinifera inhibits LPS-induced inflammatory mediators in murine macrophage. Toxicol Res. 26: 37–46 (2010).
Jeong MH, Yang KM, Kim JM, Nam BH, Kim GY, Lee SW, Seo SY, Jo WS. Inhibitory effects of Asterina pectinifera extracts on melanin biosynthesis through tyrosinase activity. Int J Mol Med. 31: 205–212 (2013).
Nam KS, Shon YH. Chemopreventive effects of polysaccharides extract from Asterina pectinifera on HT-29 human colon adenocarcinoma cells. BMB Rep. 42: 277–280 (2009).
Yang CH, Kao YH, Huang KS, Wang CY, Lin LW. Cordyceps militaris and mycelial fermentation induced apoptosis and autophagy of human glioblastoma cells. Cell Death Dis. 3: e431 (2012).
Stone R. Last stand for the body snatcher of the himalayas? Science 322: 1182 (2008).
Stone R. Improbable partners aim to bring biotechnology to a himalayan kingdom. Science 327: 940–941 (2010).
Tuli HS, Sharma AK, Sandhu SS. Optimization of fermentation conditions for cordycepin production using Cordyceps militaris 3936. JBCS 1: 35–47 (2014).
Yoshikaw N, Nakamura K, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumour activity of cordycepin in mice. Clin Exp Pharmacol Physiol. 31: S51–S53 (2004).
Chen Y, Chen YC, Lin YT, Huang SH, Wang SM. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J Agric Food Chem. 58: 11645–11652 (2010).
Wehbe-Janek H, Shi Q, Kearney CM. Cordycepin/hydroxyurea synergy allows low dosage efficacy of cordycepin in MOLT-4 leukemia cells. Anticancer Res. 27: 3143–3146 (2007).
Xu HL, Zhang LJ, Shi H, Zhu X, He X. Effects of cordycepin on Hep G2 and EA. hy926 cells: potential antiproliferative, antimetastatic and anti-angiogenic effects on hepatocellular carcinoma. Oncol Lett. 7: 1556–62 (2014).
Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol. 96: 555–561 (2005).
Tang YJ, Zhong JJ. Fed-batch fermentation of Ganoderma lucidum for hyperproduction of polysaccharide and ganoderic acids. Enzyme Microb Tech. 31: 20–8 (2002).
Park JP, Kim SW, Hwang HJ, Yun JW. Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett App Microb. 33: 76–81 (2001).
Fang QH, Zhong JJ. Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites—ganoderic acid and polysaccharide. Biochem Eng J. 10: 61–5 (2002).
Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 63: 11–20 (2014).
Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 10: S76–S83 (2014).
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105: 141–150 (2014).
Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D’Acierno L, Giordano R, Palma GD, Conte M, Golino P, Russo MG, Calabrò R, Calabrò P. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 16: 1–7 (2014).
Li H, Yoon JH, Won HJ, Ji HS, Yuk HJ, Park KH, Park HY, Jeong TS. Isotrifoliol inhibits pro-inflammatory mediators by suppression of TLR/NF-κB and TLR/MAPK signaling in LPS-induced RAW264.7 cells. Int Immunopharmacol. 45: 110–119 (2017).
Abarikwu SO. Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-kappaB, p38, Akt, p-c-JUN and JNK. Biochim Biophys Acta. 1840: 2373–2381 (2014).
Park PJ, Je JY, Kim SK. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr Polym. 55: 17–22 (2004).
Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 282: 1075–1079 (2001).
**e QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256: 225–228 (1992).
Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of PKC alpha/beta in TLR4 and TLR2 dependent activation of NF-κB. Cell Signal. 17: 385–394 (2005).
Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 17: 1–14 (2005).
Kim YS, Kim EK, Natarajan SB, Hwang JW, Kim SE, Jeon NJ, Lee JW, Jeong JH, Kim HJ, Park PJ. Radical scavenging activities of Asterina pectinifera fermented with Cordyceps militaris mycelia. Food Sci Biotechnol. 25(S): 97–101 (2016).
Duque GA, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Frontiers in Immunol. 5(491): 1–12 (2014).
Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signaling pathways. Biochem Soc Trans. 29: 345–350 (2001).
Kao SJ, Lei HC, Kuo CT, Chang MS, Chen BC, Chang YC, Chiu WT, Lin CH. Lipoteichoic acid induces nuclear factor-kappa B activation and nitric oxide synthase expression via phosphatidylinositol 3-kinase, Akt, and p38 MAPK in RAW 264.7 macrophages. Immunol. 115: 366–374 (2005).
Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 121: 2357–2363 (2007).
Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 24: 96–102 (2003).
Jung CH, Jung H, Shin YC, Park JH, Jun CY, Kim HM, Yim HS, Shin MG, Bae HS, Kim SH, Ko SG. Eleutherococcus senticosus extract attenuates LPS-induced iNOS expression through the inhibition of Akt and JNK pathways in murine macrophage. J Ethnopharmacol. 113: 183–187 (2007).
Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clinica Chimica Acta. 412: 513–520 (2011).
Acknowledgements
This research was a part of the project, the Development and Industrialization of Organism Defense Regulatory Materials from Extracts of Starfish Fermented with Cordyceps militaris, funded by the Ministry of Oceans and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, YS., Shin, WB., Dong, X. et al. Anti-inflammatory effect of the extract from fermented Asterina pectinifera with Cordyceps militaris mycelia in LPS-induced RAW264.7 macrophages. Food Sci Biotechnol 26, 1633–1640 (2017). https://doi.org/10.1007/s10068-017-0233-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0233-9