Abstract
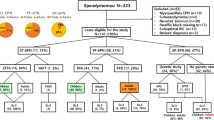
We focused on histological and immunohistochemical characteristics of ependymoma (EPN) with molecular profiles to develop more reproducible criteria of the diagnosis. Three expert neuropathologists reviewed the pathology of 130 samples from the Japan Pediatric Molecular Neuro-Oncology Group study. Confirmed cases were assessed for histology, surrogate markers, molecular subgrou**, and survival data. We reached a consensus regarding the diagnosis of EPNs in 100% of spinal cord tumors and 93% of posterior fossa (PF) tumors that had been diagnosed as EPNs by local pathologists, whereas we reached a consensus regarding only 77% of the local diagnosis of supratentorial (ST) EPNs. Among the PF-EPNs, most of anaplastic ependymomas (AEPNs) were defined as EPN-A by methylation profiling, which was significantly correlated with the subgroup assignment. Regarding prognosis, the overall survival of patients with PF-EPN was significantly better than that of patients with PF AEPN (p = 0.01). Histologically, all ependymoma, RELA fusion-positive (EPN-RELA) qualified as Grade III. Both L1 cell adhesion molecule and nuclear factor kappaB p65 antibodies showed good sensitivity for detecting EPN-RELA. This study indicated that the expert consensus pathological diagnosis could correlate well with the molecular classifications in EPNs. ST EPNs should be diagnosed more carefully by histological and molecular analyses.




Similar content being viewed by others
References
Ellison DW, McLendon R, Wiestler OD et al (2016) Ependymoma. In: Louis DN, Ohgaki H. Wiestler OD, Cavenee WK (eds) WHO classification of tumours of the central nervous system. Fourth Edition revised. IARC press, Lyon, pp 106–111
Healey EA, Barnes PD, Kupsky WJ et al Scott RM, Sallan SE, Black PM, Tarbell NJ (1991) The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery 28:666–671
Ikezaki K, Matsushima T, Inoue T et al (1993) Correlation of microanatomical localization with postoperative survival in posterior fossa ependymomas. Neurosurgery 32:38–44
Pollack IF, Gerszten PC, Martinez AJ et al (1995) Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery 37:655–666
van Veelen-Vincent ML, Pierre-Kahn A, Kalifa C et al (2002) Ependymoma in childhood: prognostic factors, extent of surgery, and adjuvant therapy. J Neurosurg 97:827–835
Metellus P, Barrie M, Figarella-Branger D et al (2007) Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain 130:1338–1349
Ellison DW, Kocak M, Figarella-Branger D et al (2011) Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negative Results Biomed 10:7
Pajtler K, Mack SC, Ramaswamy V et al (2017) The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variant. Acta Neuropathol 133:5–12
Acquaye A, Vera E, Gilbert MR et al (2017) Clinical presentation and outcomes for adult ependymoma patients. Cancer 123:494–501
Parker M, Mohankumar KM, Punchihewa C et al (2014) C11orf95-RELA fusions drive oncogenic NF-κB signallimng in ependymoma. Nature 506:451–455
Pajtler KW, Witt H, Sill M et al (2015) Molecular classification of ependymal tumors across all CNS compartments. Histopathological grades, and age groups. Cancer Cell 27:728–743
Figarella-Branger D, Lachapt-Zalcman E, Tobouret E et al (2016) Supratentorial clear cell ependymomas with branching capillaries demonstrate characteristic clinicopathological features and pathological activation of nuclear factor-kappaB signaling. Neuro-Oncol 18:919–927
Nakamura T, Fukuoka K, Ikeda J et al (2017) Encouraging option of multi-staged gross total resection for a C11orf-RelA fusion-positive supratentorial anaplastic ependymoma. Brain Tumor Pathol 34:160–164
Onishi S, Yamasaki F, Nakano Y et al (2018) RELA fusion-positive anaplastic ependymoma: molecular characterization and advanced MR imaging. Brain Tumor Pathol 35:41–45
Witt H, Mack SC, Ryzhova M et al (2011) Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20:143–157
Sasaki A, Yokoo H, Tanaka Y et al (2013) Characterization of microglia/macrophages in gliomas developed in S-100βv-erbB transgenic rats. Neuropathology 33:505–514
Fukuoka K, Kanemura Y, Shofuda T et al (2018) Significance of molecular classification in ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogenous group of tumor. Acta Neuropathol Commun 6(1):134. https://doi.org/10.1186/s40478-018-0630-1
Wani K, Armstrong TS, Vera-Bolanos E et al (2012) Collaborative Ependymoma Research Network. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123:727–738
Hoffman LM, Donson AM, Nakachi I et al (2014) Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol 127:731–745
Tihan T, Zhou T, Holmes E et al (2008) The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol 21:165–177
Rezai AR, Woo HH, Lee M et al (1996) Disseminated ependymomas of the central nervous system. J Neurosurg 85:618–624
Prayson RA (1998) Cyclin D1 and MIB-1 immunohistochemistry in ependymomas: a study of 41 cases. Am J Clin Pathol 110:629–634
Figarella-Branger D, Civatte M, Bouvier-Labit C et al (2000) Prognostic factors in intracranial ependymomas in children. J Neurosurg 93:605–613
Wolfsberger S, Fischer I, Hoftberger R et al (2004) Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am J Surg Pathol 28:914–920
Kurt E, Zheng PP, Hop WC et al (2006) Identification of relevant prognostic histopathologic features in 69 intracranial ependymomas, excluding myxopapillary ependymomas and subependymomas. Cancer 106:388–395
Araki A, Chocholous M, Gojo J et al (2016) Chromosome 1q gain ad tenascin-C expression are candidate markers to define different risk groups in pediatric posterior fossa ependymoma. Acta Neuropathol Commun 4:88–97
Bayliss J, Mukherjee P, Lu C et al (2016) Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci Transl Med 8:366ra161
Panwalkar P, Clark J, Ramaswamy V et al (2017) Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol 134:705–714
Tsuzuki T, Izumoto S, Ohnishi T et al (1998) Neural cell adhesion molecule L1 in gliomas: correlation with TGF-β. J Clin Pathol 51:13–17
Suzuki T, Izumoto S, Fujimoto Y et al (2005) Clinicopathological study of cellular proliferation and invasion in gliomatosis cerebri: important role of neural cell adhesion molecule L1 in tumour invasion. J Clin Pathol 58:166–171
Pietsch T, Wohlers I, Goschzik T et al (2014) Supratentorial epoendymomas of childfood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signalling pathway. Acta Neuropathol 127:609–611
Wang H, Wang H, Zhang W et al (2004) Analysis of the activation status of Akt, NFκB, and Stat3 in human diffuse gliomas. Lab Invest 84:941–951
Min KW, Scheithauer BW (1997) Clear cell ependymoma: a mimic of oligodendroglioma: clinicopathologic and ultrastructural considerations. Am J Surg Pathol 21:820–826
Fouladi M, Helton K, Dalton J et al (2003) Clear cell ependymoma: a clinicopathologic and radiographic analysis of 10 patients. Cancer 98:2232–2244
Tihan T, Burger PC (2016) Ependymoma. In: Kleinschmidt-deMasters BK, Rodriguez FJ, Tihan T (eds) Diagnostic pathology neuropathology, 2 edn. Elsevier, Inc, Salt Lake City
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Liu Z, Li J, Liu Z et al (2014) Supratentorial cortical ependymoma: case series and review of the literature. Neuropathology 34:243–252
Acknowledgements
We thank the following individuals for their comments regarding pathological diagnosis: Takashi Komori, Makoto Shibuya, Hiroyoshi Suzuki, and Shinya Tanaka. The authors also thank Tomio Honma and Toshinori Nagai for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sasaki, A., Hirato, J., Hirose, T. et al. Review of ependymomas: assessment of consensus in pathological diagnosis and correlations with genetic profiles and outcome. Brain Tumor Pathol 36, 92–101 (2019). https://doi.org/10.1007/s10014-019-00338-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-019-00338-x