Abstract
Context
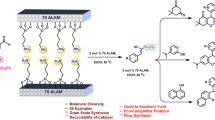
In this work, a series of heterocyclic alkenes were prepared by the reaction of 2-hydroxy-1-naphthaldehyde with various heterocyclic active methylene compounds via Knoevenagel condensation reaction using mesoporous silica, MCM 41, supported perchloric acid as an efficient green catalytic system under solvent-free conditions. A comparative study of the conventional method vs the green method was also reported with the same raw materials. 1H NMR, 13C NMR, IR, and mass spectroscopic techniques were used for the characterization of synthesized compounds.
Methods
Computational study was performed for these compounds by applying density functional theory (DFT) at M06 functional and 6-311G (d,p) basis set to interpret the electronic structures and counter check the experimental findings. The frequency analysis with aforementioned levels of DFT was performed to confirm the stability associated with optimized geometries. The true minimum for the optimized geometries for 1, 2, and 3 was achieved as indicated by the absence of negative eigenvalues in all the calculated frequencies. Additionally, natural bond orbitals (NBOs) and nonlinear optical (NLO) properties were explored utilizing the aforementioned level and basis set combination via DFT, whereas the frontier molecular orbitals (FMOs) evaluation was done at time-dependent density functional theory TDDFT at M06/6-311G(d,p). The global reactivity parameters were also calculated using the FMO data. These computation-based outcomes were found in good agreement with the experimental findings.





Similar content being viewed by others
References
Anastas PT, Warner JC (1998) Green Chemistry: Theory and Practice. Oxford Univ Press, Oxford, p 160
Tarannum S, Siddiqui ZN (2015) Fe (OTs)3/SiO2: a novel catalyst for the multicomponent synthesis of dibenzodiazepines under solvent-free conditions. RSC Adv 5(91):74242–74250. https://doi.org/10.1039/C5RA12085C
Vidyacharan S, Shinde AH, Satpathi B, Sharada DS (2014) A facile protocol for the synthesis of 3-aminoimidazo-fused heterocycles via the Groebke-Blackburn-Bienayme reaction under catalyst-free and solvent-free conditions. Green Chem 16(3):1168–1175. https://doi.org/10.1039/C3GC42130A
Kokel A, Schäfer C, Török B (2017) Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem 19(16):3729–3751
Siddiqui ZN, Khan K, Ahmed N (2014) Nano fibrous silica sulphuric acid as an efficient catalyst for the synthesis of β-enaminone. Catal Lett 144(4):623–632. https://doi.org/10.1007/s10562-013-1190-4
Tale RH, Rodge AH, Hatnapure GD, Keche AP (2011) The novel 3, 4-dihydropyrimidin-2 (1H)-one urea derivatives of N-aryl urea: synthesis, anti-inflammatory, antibacterial and antifungal activity evaluation. Bioorg Med Chem Lett 21(15):4648–4651. https://doi.org/10.1016/j.bmcl.2011.03.062
Ghorab MM, El-Gazzar MG, Alsaid MS (2014) Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles. Int J Mol Sci 15(5):7539–7553. https://doi.org/10.3390/ijms15057539
Kim J, Park C, Ok T, So W, Jo M, Seo M, Kim Y, Sohn JH, Park Y, Ju MK, Kim J (2012) Discovery of 3, 4-dihydropyrimidin-2 (1H)-ones with inhibitory activity against HIV-1 replication. Bioorg Med Chem Lett 22(5):2119–2124. https://doi.org/10.1016/j.bmcl.2011.12.090
Ellis GP, Becket GJ, Shaw D, Wilson HK, Vardey CJ, Skidmore IF (1978) Benzopyrones. 14. Synthesis and anti-allergic properties of some N-tetrazolylcarboxamides and related compounds. J Med Chem 21(11):1120–1126. https://doi.org/10.1021/jm00209a006
Chen YF, Wu SN, Gao JM, Liao ZY, Tseng YT, Fülöp F, Chang FR, Lo YC (2020) The antioxidant, anti-inflammatory, and neuroprotective properties of the synthetic chalcone derivative. AN07. Molecules 25(12):2907. https://doi.org/10.3390/molecules25122907
Thapa P, Upadhyay SP, Suo WZ, Singh V, Gurung P, Lee ES, Sharma R, Sharma M (2021) Chalcone and its analogs: therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg Chem 108:104681. https://doi.org/10.1016/j.bioorg.2021.104681
Hałdys K, Latajka R (2019) Thiosemicarbazones with tyrosinase inhibitory activity. Med Chem Comm 10(3):378–389. https://doi.org/10.1039/C9MD00005D
Siddiqui ZN, Ahmed N, Farooq F, Khan K (2013) Highly efficient solvent-free synthesis of novel pyranyl pyridine derivatives via β-enaminones using ZnO nanoparticles. Tetrahedron Lett 54(28):3599–3604. https://doi.org/10.1016/j.tetlet.2013.04.072
Dong DJ, Li HH, Tian SK (2010) A highly tuneable stereo selective olefination of semi stabilized triphenylphosphonium ylides with N-sulfonyl imines. J Am Chem Soc 132(14):5018–5020. https://doi.org/10.1021/ja910238f
Kim S, Seus P, Meier H (2004) Synthesis of tetrastilbenylmethanes by Wittig–Horner reactions. Eur J Org Chem 2004(8):1761–1764. https://doi.org/10.1002/ejoc.200300707
Kofoed J, Reymond JL, Darbre T (2005) Prebiotic carbohydrate synthesis: zinc–proline catalyzes direct aqueous aldol reactions of α-hydroxy aldehydes and ketones. Org Biomol Chem 3(10):1850–1855. https://doi.org/10.1039/B501512J
Reddy K, Raja Sekhar CV, Krishna GG (2007) Zinc–proline complex: an efficient, reusable catalyst for direct nitroaldol reaction in aqueous media. Synthetic Commun 37(12):1971–1976. https://doi.org/10.1080/00397910701354731
Assadi N, Pogodin S, Agranat I (2011) Peterson olefination: unexpected rearrangement in the overcrowded polycyclic aromatic ene series. Eur J Org Chem 2011:6773–6780. https://doi.org/10.1002/ejoc.201100789
Blakemore PR (2002) The modified Julia olefination: alkene synthesis via the condensation of metallated heteroarylalkylsulfones with carbonyl compounds. J Chem Soc Perkin Trans 1(23):2563–2585. https://doi.org/10.1039/B208078H
Abraham S, Sundararajan G (2006) Investigation of the active species in a Michael addition promoted by chirally modified tetrahydroborate. Tetrahedron 62(7):1474–1478. https://doi.org/10.1016/j.tet.2005.11.023
Kidwai M, Jain A, Poddar R, Bhardwaj S (2011) Bis [(L) prolinato-N, O] Zn in acetic acid–water: a novel catalytic system for the synthesis of β-amino carbonyls via Mannich reaction at room temperature. Appl Organomet Chem 25(5):335–340. https://doi.org/10.1002/aoc.1764
Ryabukhin SV, Plaskon AS, Volochnyuk DM, Pipko SE, Shivanyuk AN, Tolmachev AA (2007) Combinatorial Knoevenagel reactions. J Comb Chem 9(6):1073–1078. https://doi.org/10.1021/cc070073f
Siddiqui ZN, Khan T (2013) P2O5/SiO2 as an efficient heterogeneous catalyst for the synthesis of heterocyclic alkene derivatives under thermal solvent-free conditions. Catal. Sci Technol 3(8):2032–2043. https://doi.org/10.1039/C3CY00095H
Kumar SV, Banerjee S, Punniyamurthy T (2020) Transition metal-catalyzed coupling of heterocyclic alkenes via C–H functionalization: recent trends and applications. Org Chem Front 7(12):1527–1569. https://doi.org/10.1039/D0QO00279H
Sans V, Gelat F, Karbass N, Burguete MI, García-Verdugo E, Luis SV (2010) Polymer cocktail: a multitask supported ionic liquid-like species to facilitate multiple and consecutive C=C coupling reactions. Adv Synth Catal 352(17):3013–3021. https://doi.org/10.1002/adsc.201000528
Fournier D, Hoogenboom R, Schubert US (2007) Clicking polymers: a straightforward approach to novel macromolecular architectures. Chem Soc Rev 36(8):1369–1380. https://doi.org/10.1039/B700809K
Parida KM, Rath D (2009) Amine functionalized MCM-41: an active and reusable catalyst for Knoevenagel condensation reaction. J Mol Catal A Chem 310(1-2):93–100. https://doi.org/10.1016/j.molcata.2009.06.001
Grün M, Lauer I, Unger KK (1997) The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv Mater 9(3):254–257. https://doi.org/10.1002/adma.19970090317
Kyriakopoulos J, Papastavrou AT, Panagiotou GD, Tzirakis MD, Triantafyllidis KS, Alberti MN, Bourikas K, Kordulis C, Orfanopoulos M, Lycourghiotis A (2014) Deposition of fullerene C60 on the surface of MCM-41 via the one-step wet impregnation method: active catalysts for the singlet oxygen mediated photooxidation of alkenes. J Mol Catal A Chem 381:9–15. https://doi.org/10.1016/j.molcata.2013.09.036
Das D, Lee JF, Cheng S (2001) Sulfonic acid functionalized mesoporous MCM-41 silica as a convenient catalyst for Bisphenol-A synthesis. Chem Commun 21:2178–2179. https://doi.org/10.1039/B107155F
Martins L, Hölderich W, Hammer P, Cardoso D (2010) Preparation of different basic Si–MCM-41 catalysts and application in the Knoevenagel and Claisen–Schmidt condensation reactions. J Catal 271(2):220–227. https://doi.org/10.1016/j.jcat.2010.01.015
Wu C, Dong L, Onwudili J, Williams PT, Huang J (2013) Effect of Ni particle location within the mesoporous MCM-41 support for hydrogen production from the catalytic gasification of biomass. ACS Sustain Chem Eng 1(9):1083–1091. https://doi.org/10.1021/sc300133c
Rostamizadeh S, Nojavan M, Aryan R, Isapoor E, Azad M (2013) Amino acid-based ionic liquid immobilized on α-Fe2O3-MCM-41: an efficient magnetic nano catalyst and recyclable reaction media for the synthesis of quinazolin-4 (3H)-one derivatives. J Mol Catal A Chem 374:102–110. https://doi.org/10.1016/j.molcata.2013.04.002
Subramanian T, Kumarraja M, Pitchumani K (2012) Al-MCM-41 as a mild and ecofriendly catalyst for Michael addition of indole to α, β-unsaturated ketones. J Mol Catal A Chem 363:115–121. https://doi.org/10.1016/j.molcata.2012.05.024
Ray S, Brown M, Bhaumik A, Dutta A, Mukhopadhyay C (2013) A new MCM-41 supported HPF 6 catalyst for the library synthesis of highly substituted 1, 4-dihydropyridines and oxidation to pyridines: report of one-dimensional packing towards LMSOMs and studies on their photophysical properties. Green Chem 15(7):1910–1924. https://doi.org/10.1039/C3GC40441B
Khan K, Siddiqui ZN (2015) MCM-41 supported perchloric acid for efficient synthesis of 3, 4-dihydropyrimidin-2-(1H)-ones via Biginelli reaction. Monatshfür Chem 146(12):2097–2105. https://doi.org/10.1007/s00706-015-1468-x
Shinde RA, Adole VA, Shinde RS, Desale BS, Jagdale BS (2022) Synthesis, antibacterial, antifungal and computational study of (E)-4-(3-(2, 3-dihydrobenzo [b][1, 4] dioxin-6-yl)-3-oxoprop-1-en-1-yl) benzonitrile. Results Chem 4:100553
Shinde RA, Adole VA, Jagdale BS, Pawar TB (2020) Experimental and theoretical studies on the molecular structure, FT-IR, NMR, HOMO, LUMO, MESP, and reactivity descriptors of (E)-1-(2, 3-dihydrobenzo [b][1, 4] dioxin-6-yl)-3-(3, 4, 5-trimethoxyphenyl) prop-2-en-1-one. Mater Sci Res India 17:54–72
Adole VA, More RA, Shinde RA, Dhonnar SL, Jagdale BS, Shinde SG, Patil AV, Pawar TB (2021) Spectroscopic (FTIR and UV), quantum chemical, antifungal and antioxidant investigations of (E)-7-(4-(trifluoromethyl) benzylidene)-1, 2, 6, 7-tetrahydro-8H-indeno [5, 4-b] furan-8-one: a combined experimental and theoretical study. Vietnam J Chem 59(5):689–700
Shinde RA, Adole VA, Jagdale BS (2022) Synthesis, computational, antibacterial and antifungal investigation of two tri-fluorinated chalcones of 1-(2, 3-dihydrobenzo [b][1, 4] dioxin-6-yl) ethan-1-one. Polycycl Aromat Compd 42(9):6155–6172. https://doi.org/10.1080/10406638.2021.1977346
Shinde RA, Adole VA, Jagdale BS, Desale BS (2021) Synthesis, antibacterial and computational studies of Halo Chalcone hybrids from 1-(2, 3-dihydrobenzo [b][1, 4] dioxin-6-yl) ethan-1-one. J Indian Chem Soc 98(4):100051. https://doi.org/10.1016/j.jics.2021.100051
Shinde RA, Adole VA, Jagdale BS (2021) Antimicrobial and computational investigation of two 2, 3-dihydro-1 H-inden-1-one derived fluorinated chalcone motifs. Vietnam J Chem 59(6):800–812
Günay N, Pir HA, Avcı D, Atalay Y (2013) NLO and NBO analysis of sarcosine-maleic acid by using HF and B3LYP calculations. J Chem 2013:1–16
Khalid M, Ullah MA, Adeel M, Khan MU, Tahir MN, Braga AAC (2019) Synthesis, crystal structure analysis, spectral IR, UV–Vis, NMR assessments, electronic and nonlinear optical properties of potent quinoline based derivatives: interplay of experimental and DFT study. J Saudi Chem Soc 23(5):546–560. https://doi.org/10.1016/j.jscs.2018.09.006
Khalid M, Lodhi HM, Khan MU, Imran M (2021) Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: a DFT approach. RSC Adv 11(23):14237–14250. https://doi.org/10.1039/D1RA00876E
Khalid M, Khan MU, Hussain R, Irshad S, Ali B, Braga AAC, Imran M, Hussain A (2021) Exploration of second and third order nonlinear optical properties for theoretical framework of organic D–π–D–π–A type compounds. Opt Quantum Electron 53(10):1–9. https://doi.org/10.1007/s11082-021-03212-3
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge University Press
Khalid M, Ali A, Rehman MF, Mustaqeem M, Ali S, Khan MU, Asim S, Ahmad N, Saleem M (2020) Exploration of noncovalent interactions, chemical reactivity, and nonlinear optical properties of piperidone derivatives: a concise theoretical approach. ACS Omega 5(22):13236–13249. https://doi.org/10.1021/acsomega.0c01273
Khalid M, Arshad MN, Murtaza S, Shafiq I, Haroon M, Asiri AM, de AlcântaraMorais SF, Braga AAC (2022) Enriching NLO efficacy via designing non-fullerene molecules with the modification of acceptor moieties into ICIF2F: an emerging theoretical approach. RSC Adv 12(21):13412–13427. https://doi.org/10.1039/D2RA01127A
Khalid M, Khan MU, Razia ET, Shafiq Z, Alam MM, Imran M, Akram MS (2021) Exploration of efficient electron acceptors for organic solar cells: rational design of indacenodithiophene based non-fullerene compounds. Sci Rep 11(1):1–5. https://doi.org/10.1038/s41598-021-99254-4
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica 1(1-6):104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci 83(22):8440–8441. https://doi.org/10.1073/pnas.83.22.8440
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516. https://doi.org/10.1021/ja00364a005
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107(9):46–74. https://doi.org/10.1021/cr078014b
Khalid M, Ali A, Jawaria R, Asghar MA, Asim S, Khan MU, Hussain R, urRehman MF, Ennis CJ, Akram MS (2020) First principles study of electronic and nonlinear optical properties of A–D–π–A and D–A–D–π–A configured compounds containing novel quinoline–carbazole derivatives. RSC adv 10(37):22273–22283. https://doi.org/10.1039/D0RA02857F
Khalid M, Khan MU, Shafiq I, Hussain R, Mahmood K, Hussain A, Jawaria R, Hussain A, Imran M, Assiri MA, Ali A (2021) NLO potential exploration for D–π–A heterocyclic organic compounds by incorporation of various π-linkers and acceptor units. Arab J Chem 14(8):103295. https://doi.org/10.1016/j.arabjc.2021.103295
Khalid M, Khan MU, Shafiq I, Hussain R, Ali A, Imran M, Braga AAC, Fayyazur Rehman M, Akram MS (2021) Structural modulation of π-conjugated linkers in D–π–A dyes based on triphenylaminedicyanovinylene framework to explore the NLO properties R. Soc Open Sci 8(8):210570. https://doi.org/10.1098/rsos.210570
Khalid M, Jawaria R, Khan MU, Braga AAC, Shafiq Z, Imran M, Zafar HM, Irfan A (2021) Synthesis, characterization, and DFT-based electronic and nonlinear optical properties of methyl 1-(arylsulfonyl)-2-aryl-1H-benzo[d]imidazole-6-carboxylates. ACS Omega 6(24):16058–16065. https://doi.org/10.1021/acsomega.1c01938
Khalid M, Ali A, De la Torre AF, Marrugo KP, Concepcion O, Kamal GM, Muhammad S, Al-Sehemi AG (2020) Facile synthesis, spectral (IR, mass, UV–Vis, NMR), linear and nonlinear investigation of the novel phosphonate compounds: a combined experimental and simulation study. Chemistry Select 5(10):2994–3006. https://doi.org/10.1002/slct.201904224
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji HH, Li H, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian, Inc., Wallingford CT, GaussView 5.0. Wallingford, E.U.A.
Dennington RD, Keith TA, Millam JM (2008) GaussView 5.0. Gaussian Inc, Wallingford, p 20
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Com Chem 29(5):839–845. https://doi.org/10.1002/jcc.20823
Zhurko GA, Zhurko DA (2009) ChemCraft, version 1.6. URL http://www.chemcraftprog.com
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Chem Inform 4(1):1–7. https://doi.org/10.1186/1758-2946-4-17
Zhao Y, Truhlar DG (2008) Construction of a generalized gradient approximation by restoring the density-gradient expansion and enforcing a tight Lieb–Oxford bound. J Chem Phys 128(18):184109. https://doi.org/10.1063/1.2912068
Acknowledgements
One of the authors Ms. Snigdha K would like to acknowledge CSIR (Council of Scientific and Industrial Research), New Delhi, India, for the financial assistance in the form of Junior Research Fellowship (File No: 08/738 (0001)/2019-EMR-I). The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, has funded this project, under grant no. (KEP-64-130-42).
Author information
Authors and Affiliations
Contributions
SK (conceptualization; methodology; formal analysis; investigation; writing—original draft), MMTN (conceptualization; methodology; formal analysis; investigation; supervision; writing—review and editing), AMA (resources, methodology, formal analysis, writing—review and editing), KAA-A (resources, methodology, formal analysis, writing—review and editing), MA (resources, methodology, formal analysis, writing—review and editing). All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not acceptable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1.53 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
K., S., T. N., M.M., Asiri, A.M. et al. Green synthesis of heterocyclic alkenes using MCM 41 supported perchloric acid catalytic system: characterization and DFT studies. J Mol Model 29, 244 (2023). https://doi.org/10.1007/s00894-023-05635-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05635-z