Abstract
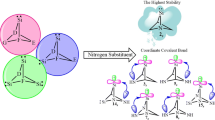
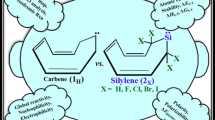
Substitution effects on stability (ΔΕs-t) of novel singlet and triplet forms of bicyclo[2.2.1]hepta-7-silylenes are compared and contrasted, at B3LYP/6–311++G** level of theory. All species appear as ground state minima on their energy surface, for showing no negative force constant. Singlets (1s-24s) are ground state and more stable than their corresponding triplets (1t-24t). The most stable scrutinized silylenes appear to be 2,3-disilabicyclo[2.2.1]hepta-7-silylene (9) for showing the highest value of ΔEs-t. This stability can be related to our imposed topology and β-silicon effect. The band gaps (ΔΕHOMO–LUMO) show the same trend as ΔEs-t and the lowest unoccupied molecular orbital energies. Also, the electrophilicity appears inverse correlation with our results of ΔΕs–t. The purpose of the present work was to assess the influence of 1 to 6 silicon substitutions on the stability, band gaps, nucleophilicity, electrophilicity, and proton affinity. Finally, our investigation introduces novel silylenes with possible applications in chemistry such as semiconductors, cumulated multidentate ligands, etc.
Graphical abstract

Synopsis
Substitution effects on stability (ΔΕs-t) of novel singlet (s) and triplet (t) forms of bicyclo[2.2.1]hepta-7-silylenes are compared and contrasted, at B3LYP/6–311++G** level of theory. All species appear as ground state minima on their energy surface, for showing no negative force constant. Singlets (1s–24s) are ground state and more stable than their corresponding triplets (1t–24t). The most stable scrutinized silylenes appear to be 2,3-disilabicyclo[2.2.1]hepta-7-silylene (9) for showing the highest value of ΔEs-t. This stability can be related to our imposed topology and β-silicon effect. The purpose of the present work was to assess the influence of 1 to 6 silicon substitutions on stability (ΔΕs–t), band gaps (ΔΕHOMO–LUMO), nucleophilicity (N), electrophilicity (ω), and proton affinity (ΔΕPA). Finally, this new generation has the intrinsic potential to form accumulated multidentate ligands.


Similar content being viewed by others
References
Ashenagar S, Kassaee MZ (2018). Turk. J. Chem. 42(4):974–987
Schoeller WW, Sundermann A, Reiher M (1999). Inorg. Chem. 38:29–37
Becerra R, Walsh R (2010). Dalt. Trans. 39:9217–9228
Mizuhata Y, Sasamori T, Tokitoh N (2009). Chem. Rev. 109:3479–3511
Tokitoh N, Okazaki R (2000). Coord. Chem. Rev. 210:251–277
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000). Chem. Rev. 100:39–92
Barden CJ, Schaefer HF (2000). J. Chem. Phys. 112:6515–6516
Lee EPF, Dyke JM, Wright TG (2000). Chem. Phys. Lett. 326:143–150
Bruce M (1991). Chem. Rev. 91:197–257
Ayoubi-Chianeh M, Kassaee MZ (2019). Res. Chem. Intermed. 45:4677–4691
Haaf M, Schmedake TA, West R (2000). Acc. Chem. Res. 33:704–714
Yao S, **ong Y, Driess M (2011). Organometallics 30:1748–1767
Blom B, Stoelzel M, Driess M (2013). Chem. Eur. J. 19:40
Goldberg DE, Harris DH, Lappert MF, Thomas KM (1976). J. Chem. Soc. Chem. Commun.:261
Abedini N, Kassaee MZ, Cummings PT (2020) Borasilylenes in focus: topological effects of nitrogen atoms by DFT. Silicon:1–7
Heaven MW, Metha GF, Buntine MA, Phys J (2001). Chem. A 105:1185–1196
Zachariah MR, Tsang W (1995). J. Phys. Chem. 99:5308–5318
Lucas DJ, Curtiss LA, Pople JA (1993). J. Chem. Phys. 99:6697–6703
Boudjouk P, Black E, Kumarathasan R (1991). Organometal. 10:2095–2096
Kassaee MZ, Buazar F, Soleimani-Amiri S, Mol J (2008) Struct. THEOCHEM 866:52–57
Cote DR, Van Nguyen S, Stamper AK, Armbrust DS, Tobben D, Conti RA, Lee GY (1999) IBM J. res. Dev. 43:5–38
Kassaee MZ, Najafi Z, Shakib FA, Momeni MR (2011). J. Organometal. Chem. 696:2059–2064
Tamao K, Kobayashi M, Matsuo T, Furukawa S, Tsuji H (2012). Chem. Commun. 48:1030–1032
Holthausen MC, Koch W, Apeloig Y (1999). J. Am. Chem. Soc. 121:2623–2624
Kassaee MZ, Zandi H (2012). J. Phys. Org. Chem. 25:50–57
Sekiguchi A, Tanaka T, Ichinohe M, Akiyama K, Tero-Kubota S, Am J (2003). Chem. Soc. 125:4962–4963
West R, Fink MJ, Michl J (1981). Science 214:1343–1344
B. T. Luke, J. A. Pople, M-B. Krogh-Jespersen, Y. Apeloig, M. Karni, J. Am. Chem. Soc. 1986, 108, 270–284
Kalcher J, Sax AF, Mol J (1992) Struct. THEOCHEM 253:287–302
Krogh-Jespersen K (1985). J. Am. Chem. Soc. 107:537–543
Yoshida M, Tamaoki N (2002). Organometallics 21:2587–2589
Soleimani Purlak N, Kassaee MZ (2020). J. Phys. Org. Chem.:33(6)
Yan Z, Truhlar DG (2008). Theor. Chem. Account 120:215–241
Becke AD (1988). Phys. Rev. 38:3098
Becke AD (1993). J. Chem. Phys. 98:5648–5652
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993). J. Comput. Chem. 14:1347–1363
Kassaee MZ, Ashenagar S (2018). J. Mol. Model. 24:2–11
Domingo LR, Chamorro E, Perez P (2008). J. Org. Chem. 73:4615–4624
Parr RG, Szentpaly L, Liu S (1999). J. Am. Chem. Soc. 121:1922–1924
Nyulaszi L, Belghazi A, Szetsi SK, Veszpremi T, Heinicke J (1994). THEOCHEM J. Mol. Struct. 313:73–81
Nyulaszi L, Schleyer PVR (1999). J. Am. Chem. Soc 121:6872–6875
Shimizu H, Gordon MS (1994). Organometallics 13:186–189
Lambert JB, Zhao Y (1996). J. Am. Chem. Soc. 118:7867–7868
J. Ola’h, T.Veszpre’mi, F. D. Proft, P. Geerlings, J. Phys. Chem. A 2007, 111, 10815–10823
J. Ola’h, F. De Proft, T.Veszpre’mi, P. Geerlings, J. Phys. Chem. A 2005, 109, 1608–1615
Scrocco E, Tomasi J (1973). New Concepts II 42:95–170
Parr RG, Pearson RG (1983). J. Am. Chem. Soc. 105:7512–7516
Acknowledgements
We acknowledge the support from the Tarbiat Modares University (TMU).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 1512 kb)
Rights and permissions
About this article
Cite this article
Abedini, N., Kassaee, M.Z. Substitution effects on novel bicyclo[2.2.1]hepta-7-silylenes by DFT. J Mol Model 27, 121 (2021). https://doi.org/10.1007/s00894-021-04726-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04726-z