Abstract
Objective
The objective of the study is to evaluate bone repair in rats treated with different alendronate doses.
Matherials and methods
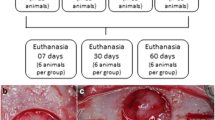
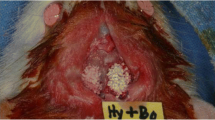
Sixty female rats ovariectomized were randomly divided in three groups: group C (control group), group A1 (ALN/1 mg/kg), and A2 (ALN/ 3 mg/kg). Each animal received subcutaneous applications of sodium alendronate at a dose correspondent to group A1 or A2 three times a week, while the control group received 0.9% saline solution. After 4 weeks of application, a critical defect was created in the calvaria of animals of all groups. The defect was filled by particulate autogenous bone. The applications were maintained until euthanasia, which occurred 15 and 60 days after the surgical procedure. The pieces were sent for histological, histomorphometric and immunohistochemical analysis. The data were submitted to statistical analysis with significance level of 0.05.
Results
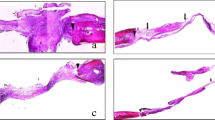
The descriptive histological analysis demonstrated an increase in bone neoformation in both groups treated with alendronate when compared to the control group. The histomorphometric analysis showed an increase in the amount of neoformed bone in A1 and A2 groups when compared to group C, both at 15 days (p = 0.0002) and at 60 days (p = 0.001). In the immunohistochemical analysis, it was possible to observe a difference in immunolabeling just for Mmp2 at the time of 60 days in A1 (p = 0.001) and A2 (p = 0.023) when compared to the control group.
Conclusion
Systemic delivery of alendronate, regardless of the dose, increased the amount of bone neoformation.
Clinical relevance
Prescription of sodium alendronate at 1 mg/kg for improvement of bone neoformation in bone graft procedures.







Similar content being viewed by others
References
Chaudhry AN, Ruggiero SL (2007) Osteonecrosis and bisphosphonates in oral and maxillofacial surgery. Oral Maxillofac Surg Clin N Am 19:199–206
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Richard P et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005 Dec;136(12):1658–68. Review. Erratum in: J Am Dent Assoc. 2006 Jan;137(1):26
Kim JH, Park YB, Li Z, Shim JS, Moon HS, Jung HS, Chung MK (2011) Effect of alendronate on healing of extraction sockets and healing around implants. Oral Dis 17:705–711
Toker H, Ozdemir H, Ozer H, Eren K (2012) A comparative evaluation of the systemic and local alendronate treatment in synthetic bone graft: a histologic and histomorphometric study in a rat calvarial defect model. Oral Surg Oral Med Oral Pathol Oral Radiol 114(5 Suppl):S146–S152
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H (2002) Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17:2237–2246
Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technology Assessment 2014; 18 (11)
Scully C, Madrid C, Bagan J (2006) Dental endosseous implants in patients on bisphosphonate therapy. Implant Dent 15:212–215
Migliorati CA, Siegel MA, Elting LS (2006) Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol 7(6):508–514
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116(5):1202–1209
De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 2004; 10(3–4):393–401
Moschouris P, Retzepi M, Petrie A, Donos N (2016) Effect of Wnt3a delivery on early healing events during guided bone regeneration. Clin Oral Implants Res 28:283–290. https://doi.org/10.1111/clr.12796
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem 31(1):177–183
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Oral Chem 274:21491–21494
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Zhao H, Bernardo MM, Osenkowski P, Sohail A, Pei D, Nagase H, Kashiwagi M, Soloway PD, DeClerck YA, Fridman R (2004) Differential inhibition of membrane type 3 (MT3)-matrix metalloproteinase (MMP) and MT1-MMP by tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3 rgulates pro-MMP-2 activation. J Biol Chem 279(10):8592–8601 Epub 2003 Dec 16
John M, Jaworski C, Chen Z, Subramanian S, Ma W, Sun F, Li D, Spector A, Carper D (2004) Matrix metalloproteinases are down-regulated in rat lenses exposed to oxidative stress. Exp Eye Res 79(6):839–846
Fernandes C, Leite RS, Lanças FM (2005) Bisfosfonatos: síntese, análises químicas e aplicações farmacológicas. Quím Nova 28(2):274–280
Buzza JA III, Einhorn T (2016) Bone healing in 2016. Clin Cases Miner Bone Metab 13(2):101–105
Riggs BL, Melton LJ III (1992) The prevention and treatment of osteoporosis. N EngI J Med 327:620–627
Sun JS, Huang YC, Tsuang YH, Chen LT, Lin FH (2001) Sintered dicalcium pyrophosphate increases bone mass in ovariectomized rats. J Biomed Mater Res 59(2):246–253
WHO technical report series, No. 843: (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. Geneva: World Health Organization
Toker H, Ozdemir H, Ozer H, Eren K (2012) Alendronate enhances osseous healing in a rat calvarial defect model. Elsevier 57:1545–1550
Siebelt M, Waarsing JH, Groen HC, Müller C, Koelewijn SJ, de Blois E, Verhaar JAN, de Jong M, Weinans H (2014) Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone 66:163–170. https://doi.org/10.1016/j.bone.2014.06.009
Boland GM, Perkins G, Hall DJ, Tuan RS (2004) Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93(6):1210–1230
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A 102:3324–3329
Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y et al (2004) Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 279:55958–55968
Nyman JS, Lynch CC, Perrien DS, Thiolloy S, O’Quinn EC, Patil CA, Bi X, Pharr GM, Mahadevan-Jansen A, Mundy GR (2011) Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J Bone Miner Res 26(6):1252–1260
De Simone E, Caggiano N, Polli M, Rolando J, Lastra Y, Gullace F et al (2013) Efecto del alendronato sobre el perfil de citoquinas y metaloproteinasas 2 y 9 en un modelo múrido de artritis experimental. Rev Colomb Reumatol 20(4):202–210
Hashimoto K, Morishige K, Sawada K, Tahara M, Shimizu S, Ogata S, Sakata M, Tasaka K, Kimura T (2007) Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem Biophys Res Commun 354(2):478–484
Hashimoto K, Morishige K, Sawada K, Tahara M, Kawagishi R, Ikebuchi Y et al (2005 Jan 15) Alendronate inhibits intraperitoneal dissemination in vivo ovarian cancer model. Cancer Res 65(2):540–545
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
de Oliveira, N., Oliveira, J., de Souza Moraes, L. et al. Bone repair in craniofacial defects treated with different doses of alendronate: a histological, histomorphometric, and immunohistochemical study. Clin Oral Invest 23, 2355–2364 (2019). https://doi.org/10.1007/s00784-018-2670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2670-0