Abstract
Introduction
Enoxaparin is widely used to prevent venous thromboembolism after orthopedic surgery and has some adverse effects, such as osteoporosis and delay in fracture healing. However, the exact mechanism delaying bone healing by enoxaparin is still unclear.
Materials and Methods
X-ray and Micro-CT scanning were performed to detect the effects of enoxaparin on bone healing at rat model of bone defeat. CCK-8 assay and flow cytometry were conducted to measure the effects of enoxaparin on bone marrow mesenchymal stem cells (BMSCs). The mRNA/protein levels of osteocalcin (OCN), runt-related transcription factor 2 (Runx2) and bone morphogenetic protein 2 (BMP2) were analyzed by real-time PCR and western blotting, respectively. Alizarin red staining was used to observe the mineralized nodules.
Results
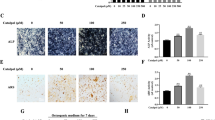
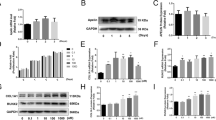
Enoxaparin (2000 AXaIU/kg) not only profoundly increased the trabecular separation, but also notably decreased the trabecular bone volume/tissue volume, trabecular thickness, trabecular number and OCN level, in vivo. Additionally, significantly inhibiting proliferation of BMSCs by enoxaparin (1.0 and 10 AXaIU/ml) was detected. The apoptosis and the ratio of G phase cells in enoxaparin (0.1, 1.0 and 10 AXaIU/ml) group were obviously higher than that in control group. While the ratio of S phase cells was downregulated markedly by enoxaparin (0.1,1.0 and 10 AXaIU/ml) compared with the control group. Most importantly, inducing significant decreases of OCN/Runx2 mRNA/protein expression and formation of mineralized nodules by enoxaparin (0.1, 1.0 and 10 AXaIU/ml) were observed compared with the control group. While the notable decreases of BMP2 mRNA/protein level were only detected in enoxaparin (10 AXaIU/ml) group.
Conclusion
It was suggested that enoxaparin impaired bone healing through suppressing the differentiation of BMSCs towards osteoblasts.





Similar content being viewed by others
References
Deitelzweig SB, Johnson BH, Lin J, Schulman KL (2011) Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 86:217–220
Jakub G, Christopher MM, Saam M et al (2014) Multiple lower-extremity and pelvic fractures increase pulmonary embolus risk. Orthopedics 37:e517-524
Bick RL, Frenkel EP, Walenga J et al (2005) Unfractionated heparin, low molecu lar weight heparins, and pentasaccharide: basic mecha nism of actions, pharmacology, and clinical use. Hematol Oncol Clin North Am 19:1–51
Matzsch T, Bergqvist D, Hedner U et al (1990) Effects of low molecular weight heparin and unfragmented heparin on induction of osteoporosis in rats. Thromb Haemost 63:505–509
Muir JM, Hirsh J, Weitz JI et al (1997) A histomorphometric comparison of the effects of heparin and low-molecular-weight heparin on cancellous bone in rats. Blood 89:3236–3242
Gajic-Veljanoski O, Phua CW, Shah PS, Cheung AM (2016) Effects of long-term low-molecular-weight heparin on fractures and bone density in non-pregnant adults: a systematic review with meta-analysis. J Gen Intern Med 31:947–957
Joshua JJ, Michael AM, Kevin JB et al (2012) American academy of orthopaedic surgeons clinical practice guideline on: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Bone J Surg Am 94:746–747
Falck-Ytter Y, Francis CW, Johanson NA et al (2012) Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of chest physicians evidence-based clinical practice guidelines. Chest 141:e278S-e325S
Fareed J, Hoppensteadt D, Walenga J et al (2003) Pharmacodynamic and pharmacokinetic properties of enoxaparin: implications for clinical practice. Clin Pharmacokinet 42:1043–1057
Tsuji K, Bandyopadhyay A, Harfe BD et al (2006) BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38:1424–1429
Luginbuehl V, Meinel L, Merkle HP et al (2004) Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm 58:197–208
Bone NA (2013) Ten Cate’s oral histology: development, structure and function. Elsevier, Rio de Janeiro, pp 109–140
Fulzele K, Riddle RC, DiGirolamo DJ et al (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319
Ducy P, Desbois C, Boyce B et al (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452
Tarantino U, Cerocchi I, Scialdoni A et al (2011) Bone healing and osteoporosis. Aging Clin Exp Res 23:62–64
Song ZH, **e W, Zhu SY et al (2018) Effects of PEMFs on Osx, Ocn, TRAP, and CTSK gene expression in postmenopausal osteoporosis model mice. Int J Clin Exp Pathol 11:1784–1790
Folwarczna J, Janiec W, Gawor M et al (2004) Effects of enoxaparin on histomorphometric parameters of bones in rats. Pol J Pharmacol 56:451–457
Folwarczna J, Sliwiński L, Janiec W, Pikul M (2005) Effects of standard heparin and low-molecular-weight heparins on the formation of murine osteoclasts in vitro. Pharmacol Rep 57:635–645
Solayar GN, Walsh PM, Mulhall KJ (2011) The effect of a new direct Factor Xa inhibitor on human osteoblasts: an in-vitro study comparing the effect of rivaroxaban with enoxaparin. BMC Musculoskelet Disord 12:247
Hirsh J, Warkentin TE, Shaughnessy SG et al (2001) Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safty. Chest 119:64S-94S
Rajgopal R, Bear M, Butcher MK, Shaughnessy SG (2008) The effects of heparin and low molecular weigh the heparins on bone. Thromb Res 122:293–298
Andrej MN, Hans D, Silvan Z et al (2013) Micro CT analysis of the subarticular bone structure in the area of the talar trochlea. Surg Radiol Anat 35:283–293
Cenni E, Perut F, Ciapetti G et al (2009) In vitro evaluation of freeze-dried bone allografts combined with platelet rich plasma and human bone marrow stromal cells for tissue engineering. J Mater Sci Mater Med 20:45–50
Calloni R, Cordero EAA, Henriques JAP, Bonatto D (2013) Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev 22:1455–1476
Kopesky PW, Lee H-Y, Vanderploeg EJ et al (2010) Adult equine bone marrow stromal cells produce a cartilage-like ECM mechanically superior to animal-matched adult chondrocytes. Matrix Biol 29:427–438
Whitfield MJ, Lee WC, Van Vliet KJ (2013) Onset of heterogeneity in culture—expanded bone marrow stromal cells. Stem Cell Res 11:1365–1377
Alturkistani A, Ghonem N, Power-Charnitsky VA et al (2019) Inhibition of PAR-1 receptor signaling by enoxaparin reduces cell proliferation and migration in A549 cells. Anticancer Res 39:5297–5310
Pilge H, Fröbel J, Mrotzek SJ et al (2016) Effects of thromboprophylaxis on mesenchymal stromal cells during osteogenic differentiation: an in-vitro study comparing enoxaparin with rivaroxaban. BMC Musculoskelet Disord 17:108
Lean QY, Patel RP, Stewart N et al (2014) Identification of pro- and anti-proliferative oligosaccharides of heparins. Integr Biol (Camb) 6:90–99
Dhoke NR, Kalabathula E, Kaushik K et al (2016) Histone deacetylases differentially regulate the proliferative phenotype of mouse bone marrow stromal and hematopoietic stem/progenitor cells. Stem Cell Res 17:170–180
Horiuchi A, Nikaido T, Ya-Li Z et al (1999) Heparin inhibits proliferation of myometrial and leiomyomal smooth muscle cells through the induction of alpha-smooth muscle actin, calponin h1 and p27. Mol Hum Reprod 5:139–145
Pilge H, Fröbel J, Prodinger PM et al (2016) Enoxaparin and rivaroxaban have different effects on human mesenchymal stromal cells in the early stages of bone healing. Bone Joint Res 5:95–100
Wang T, Wang Y, Menendez A et al (2015) Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res 30:1572–1584
Acknowledgements
This study was supported by the Foundation of Shanxi Educational Committee (Grant No. 201802057), the Youth Research Fund of Shanxi Medical University (Grant No. 02201518), and the Youth Research Fund of Shanxi Province (Grant No. 201601D021169).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, Y., Liu, L., Li, S. et al. Impaired bone healing by enoxaparin via inhibiting the differentiation of bone marrow mesenchymal stem cells towards osteoblasts. J Bone Miner Metab 40, 9–19 (2022). https://doi.org/10.1007/s00774-021-01268-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01268-5