Abstract
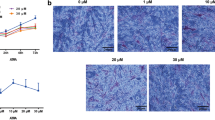
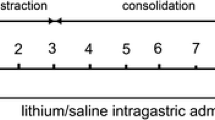
Sclerostin is a known inhibitor of the Wnt signaling pathway which is involved in osteogenesis and, when inactivated, stimulates bone formation. To our knowledge, this effect has not been studied in the context of distraction osteogenesis (DO). Tibial DO was conducted on a total of 24 wild-type mice, which were then divided into 2 groups—a saline injection group (control) and an anti-sclerostin (Scl-Ab) injection group (treatment). The mice in the treatment group received 100 mg/kg intravenous injections of the antibody weekly until killing. The 12 mice in each group were subdivided into four time points according to post-osteotomy time of killing—11 days (mid-distraction), 17 days (late distraction), 34 days (mid-consolidation) and 51 days (late consolidation), with 3 mice per subgroup. After killing, the tibia specimens were collected for immunohistochemical analysis. Our results show that the group injected with anti-sclerostin had an earlier peak (day 11) in the distraction phase of the osteogenic molecules involved in the Wnt signaling pathway in comparison to the placebo group. In addition, downregulation of the inhibitors of this pathway was noted in the treatment group when compared with the placebo group. Furthermore, LRP-5 showed a significant increase in expression in the treatment group. Sclerostin inhibition has a significant effect on the DO process through its effect on the Wnt pathway. This effect was evident through the decreased effect of sclerostin on LRP-5 and earlier upregulation of the osteogenic molecules involved in this pathway.





Similar content being viewed by others
References
Ilizarov GA (1989) The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res 238:249–281
Ilizarov GA (1989) The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res 239:263–285
Rauch F, Lauzier D, Croteau S, Travers R, Glorieux FH, Hamdy R (2000) Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone 27:453–459
Aronson J, Good B, Stewart C, Harrison B, Harp J (1990) Preliminary studies of mineralization during distraction osteogenesis. Clin Orthop Relat Res 43–49
Paley D (1990) Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res 81–104
Ghadakzadeh S, Mekhail M, Aoude A, Hamdy R, Tabrizian M (2016) Small players ruling the hard game: siRNA in bone regeneration. J Bone Miner Res 31:475–487. doi:10.1002/jbmr.2816
Makhdom AM, Hamdy RC (2013) The role of growth factors on acceleration of bone regeneration during distraction osteogenesis (in eng). Tissue Eng Part B Rev 19:442–453. doi:10.1089/ten.TEB.2012.0717
Chen Y, Alman BA (2009) Wnt pathway, an essential role in bone regeneration. J Cell Biochem 106:353–362. doi:10.1002/jcb.22020
Kasaai B, Moffatt P, Al-Salmi L, Lauzier D, Lessard L, Hamdy RC (2012) Spatial and temporal localization of WNT signaling proteins in a mouse model of distraction osteogenesis. J Histochem Cytochem 60:219–228. doi:10.1369/0022155411432010
Baron R, Hesse E (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 97:311–325. doi:10.1210/jc.2011-2332
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287. doi:10.1016/S0140-6736(10)62349-5
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P et al (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869. doi:10.1359/jbmr.080216
Li C, Ominsky MS, Tan HL, Barrero M, Niu QT, Asuncion FJ, Lee E, Liu M, Simonet WS, Paszty C, Ke HZ (2011) Increased callus mass and enhanced strength during fracture healing in mice lacking the sclerostin gene. Bone 49:1178–1185. doi:10.1016/j.bone.2011.08.012
Alzahrani MM, Rauch F, Hamdy RC (2015) Does sclerostin depletion stimulate fracture healing in a mouse model? Clin Orthop Relat Res. doi:10.1007/s11999-015-4640-z
Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, Asuncion FJ, Dwyer D, Han CY, Vlasseros F, Samadfam R, Jolette J, Smith SY, Stolina M, Lacey DL, Simonet WS, Paszty C, Li G, Ke HZ (2011) Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res 26:1012–1021. doi:10.1002/jbmr.307
Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B et al (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959. doi:10.1002/jbmr.14
Eddleston A, Marenzana M, Moore AR, Stephens P, Muzylak M, Marshall D, Robinson MK (2009) A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. J Bone Miner Res 24:1662–1671. doi:10.1359/jbmr.090403
Marenzana M, Greenslade K, Eddleston A, Okoye R, Marshall D, Moore A, Robinson MK (2011) Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum 63:2385–2395. doi:10.1002/art.30385
Wang LC, Takahashi I, Sasano Y, Sugawara J, Mitani H (2005) Osteoclastogenic activity during mandibular distraction osteogenesis. J Dent Res 84:1010–1015
McMahon MS, Fukai N (2009) Cellular response to mechanical distraction-induced skeletal remodeling. Orthopedics 32:3
Hou B, Fukai N, Olsen BR (2007) Mechanical force-induced midpalatal suture remodeling in mice. Bone 40:1483–1493. doi:10.1016/j.bone.2007.01.019
Li X, Niu QT, Warmington KS, Asuncion FJ, Dwyer D, Grisanti M, Han CY, Stolina M, Eschenberg MJ, Kostenuik PJ, Simonet WS, Ominsky MS, Ke HZ (2014) Progressive increases in bone mass and bone strength in an ovariectomized rat model of osteoporosis after 26 weeks of treatment with a sclerostin antibody. Endocrinology 155:4785–4797. doi:10.1210/en.2013-1905
Shah AD, Shoback D, Lewiecki EM (2015) Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis. Int J Womens Health 7:565–580. doi:10.2147/IJWH.S73244
Makhdom AM, Rauch F, Lauzier D, Hamdy RC (2014) The effect of systemic administration of sclerostin antibody in a mouse model of distraction osteogenesis. J Musculoskelet Neuronal Interact 14:124–130
Alam N, St-Arnaud R, Lauzier D, Rosen V, Hamdy RC (2009) Are endogenous BMPs necessary for bone healing during distraction osteogenesis? Clin Orthop Relat Res 467:3190–3198. doi:10.1007/s11999-009-1065-6
Haque T, Hamade F, Alam N, Kotsiopriftis M, Lauzier D, St-Arnaud R, Hamdy RC (2008) Characterizing the BMP pathway in a wild type mouse model of distraction osteogenesis. Bone 42:1144–1153. doi:10.1016/j.bone.2008.01.028
Tay BK, Le AX, Gould SE, Helms JA (1998) Histochemical and molecular analyses of distraction osteogenesis in a mouse model. J Orthop Res 16:636–642. doi:10.1002/jor.1100160518
Campisi P, Hamdy RC, Lauzier D, Amako M, Rauch F, Lessard ML (2003) Expression of bone morphogenetic proteins during mandibular distraction osteogenesis. Plast Reconstr Surg 111:201–208. doi:10.1097/01.PRS.0000034932.99249.34 (discussion 209–210)
Haque T, Mandu-Hrit M, Rauch F, Lauzier D, Tabrizian M, Hamdy RC (2006) Immunohistochemical localization of bone morphogenetic protein-signaling Smads during long-bone distraction osteogenesis. J Histochem Cytochem 54:407–415. doi:10.1369/jhc.5A6738.2005
Kloen P, Lauzier D, Hamdy RC (2012) Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 51:59–68. doi:10.1016/j.bone.2012.03.032
Kloen P, Doty SB, Gordon E, Rubel IF, Goumans MJ, Helfet DL (2002) Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am 84-A:1909–1918
Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, Goumans MJ (2003) BMP signaling components are expressed in human fracture callus. Bone 33:362–371
Fuss M, Ehlers EM, Russlies M, Rohwedel J, Behrens P (2000) Characteristics of human chondrocytes, osteoblasts and fibroblasts seeded onto a type I/III collagen sponge under different culture conditions. A light, scanning and transmission electron microscopy study. Ann Anat 182:303–310. doi:10.1016/S0940-9602(00)80002-3
Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446. doi:10.1172/JCI6610
Oldershaw RA (2012) Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 93:389–400. doi:10.1111/j.1365-2613.2012.00837.x
Zhong N, Gersch RP, Hadjiargyrou M (2006) Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone 39:5–16. doi:10.1016/j.bone.2005.12.008
Liu G, Vijayakumar S, Grumolato L, Arroyave R, Qiao H, Akiri G, Aaronson SA (2009) Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol 185:67–75. doi:10.1083/jcb.200810137
Augello A, De Bari C (2010) The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 21:1226–1238. doi:10.1089/hum.2010.173
Ghadakzadeh S, Kannu P, Whetstone H, Howard A, Alman BA (2016) beta-Catenin modulation in neurofibromatosis type 1 bone repair: therapeutic implications. FASEB J. doi:10.1096/fj.201500190RR
Alzahrani MM, Anam EA, Makhdom AM, Villemure I, Hamdy RC (2014) The effect of altering the mechanical loading environment on the expression of bone regenerating molecules in cases of distraction osteogenesis (in English). Front Endocrinol. doi:10.3389/fendo.2014.00214
Drake MT, Farr JN (2014) Inhibitors of sclerostin: emerging concepts. Curr Opin Rheumatol 26:447–452. doi:10.1097/BOR.0000000000000073
Compton JT, Lee FY (2014) A review of osteocyte function and the emerging importance of sclerostin. J Bone Joint Surg Am 96:1659–1668. doi:10.2106/JBJS.M.01096
Perren SM (2014) Fracture healing: fracture healing understood as the result of a fascinating cascade of physical and biological interactions. Part I. An attempt to integrate observations from 30 years AO research. Acta Chir Orthop Traumatol Cech 81:355–364
McDonald MM, Morse A, Mikulec K, Peacock L, Yu N, Baldock PA, Birke O, Liu M, Ke HZ, Little DG (2012) Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res 30:1541–1548. doi:10.1002/jor.22109
Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO (2011) Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol 359:222–229. doi:10.1016/j.ydbio.2011.08.020
Larsson S (2016) Anti-sclerostin—is there an indication? Injury 47(Suppl 1):S31–S35. doi:10.1016/S0020-1383(16)30008-0
Burgers TA, Williams BO (2013) Regulation of Wnt/beta-catenin signaling within and from osteocytes. Bone 54:244–249. doi:10.1016/j.bone.2013.02.022
Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL (2015) A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 30:216–224. doi:10.1002/jbmr.2351
Matos LL, Trufelli DC, de Matos MG, da Silva Pinhal MA (2010) Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights 5:9–20
Acknowledgements
We thank Novartis Institutes of Biomedical Research (NIBR) for the provision of the sclerostin antibody, which was generated in collaboration with MorphoSys AG. We are grateful to Guylaine Bedard for preparation of the figures. This study was supported by the Shriners of North America, The Canadian Institute for Health Research (Canada), University of Dammam (Dammam, Saudi Arabia) and King Abdulaziz University (Jeddah, Saudi Arabia).
Author information
Authors and Affiliations
Contributions
MM: Data collection, interpretation and manuscript preparation. AMM: Performed surgeries, data collection, interpretation and manuscript preparation. FR: Manuscript preparation and revision. DL: Immunohistochemical aspect and manuscript revision. MK: Immunohistochemical aspect and manuscript revision. SG: Manuscript preparation and final revision. RCH: Manuscript preparation and final revision.
Corresponding author
Ethics declarations
Conflict of interest
There were no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Alzahrani, M.M., Makhdom, A.M., Rauch, F. et al. Assessment of the effect of systemic delivery of sclerostin antibodies on Wnt signaling in distraction osteogenesis. J Bone Miner Metab 36, 373–382 (2018). https://doi.org/10.1007/s00774-017-0847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0847-2