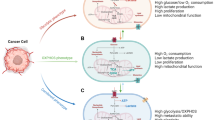
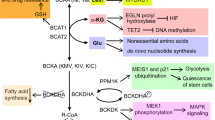
Abstract
Cancer malignancies may broadly be described as heterogeneous disorders manifested by uncontrolled cellular growth/division and proliferation. Tumor cells utilize metabolic reprogramming to accomplish the upregulated nutritional requirements for sustaining their uncontrolled growth, proliferation, and survival. Metabolic reprogramming also called altered or dysregulated metabolism undergoes modification in normal metabolic pathways for anabolic precursor’s generation that serves to continue biomass formation that sustains the growth, proliferation, and survival of carcinogenic cells under a nutrition-deprived microenvironment. A wide range of dysregulated/altered metabolic pathways encompassing different metabolic regulators have been described; however, the current review is focused to explain deeply the metabolic pathways modifications inducing upregulation of proteins/amino acids metabolism. The essential modification of various metabolic cycles with their consequent outcomes meanwhile explored promising therapeutic targets playing a pivotal role in metabolic regulation and is successfully employed for effective target-specific cancer treatment. The current review is aimed to understand the metabolic reprogramming of different proteins/amino acids involved in tumor progression along with potential therapeutic perspective elucidating targeted cancer therapy via these targets.






Similar content being viewed by others
Data availability statement
All the data has been added to this review article.
References
Andrzejewski S et al (2014) Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer & Metabolism 2(1):1–14
Bell HN et al (2022) Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 40(2):185-200.e6
Bidkhori G, Benfeitas R (2018) Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc Natl Acad Sci 115(50):E11874–E11883
Birsoy K et al (2014) Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508(7494):108–112
Birsoy K et al (2015) An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162(3):540–551
Blackhall F (2015) Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Annals Oncol 26:ii15
Brennan CA et al (2021) Fusobacterium nucleatum drives a pro-inflammatory intestinal microenvironment through metabolite receptor-dependent modulation of IL-17 expression. Gut Microbes 13(1):1987780
Burke L et al (2020) The Janus-like role of proline metabolism in cancer. Cell Death Discovery 6(1):104
Chan DA et al (2011) Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med 3(94):94ra70
Chen Y, Gao DY, Huang L (2015) In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81:128–141
Chen P et al (2022) Nanocarriers esca** from hyperacidified endo/lysosomes in cancer cells allow tumor-targeted intracellular delivery of antibodies to therapeutically inhibit c-MYC. Biomaterials 288:121748
Cluntun AA et al (2017) Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3(3):169–180
Cohen AS et al (2020) Combined blockade of EGFR and glutamine metabolism in preclinical models of colorectal cancer. Transl Oncol 13(10):100828
Comerford SA et al (2014) Acetate dependence of tumors. Cell 159(7):1591–1602
Dadwal A, Baldi A, Kumar Narang R (2018) Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol 46(Sup2):295–305
Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15(2):81–94
Dalile B et al (2019) The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol 16(8):461–478
Dang CV (2010) Rethinking the Warburg effect with myc micromanaging glutamine metabolismmyc micromanaging metabolism. Can Res 70(3):859–862
Davidson SM et al (2016) Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab 23(3):517–528
DeBerardinis RJ, Chandel NS (2016) Fundamentals of Cancer Metabolism. Sci Adv 2(5):e1600200
Dey P, Kimmelman AC, DePinho RA (2021) Metabolic codependencies in the tumor microenvironmentmetabolic codependencies in cancer. Cancer Discov 11(5):1067–1081
Eckert MA et al (2019) Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569(7758):723–728
Elgogary A et al (2016) Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci U S A 113(36):E5328–E5336
Engelman JA et al (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14(12):1351–1356
Erez A, DeBerardinis RJ (2015) Metabolic dysregulation in monogenic disorders and cancer—finding method in madness. Nat Rev Cancer 15(7):440–448
Evans JM et al (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330(7503):1304–1305
Faubert B, Solmonson A, DeBerardinis RJ (2020) Metabolic reprogramming and cancer progression. Science 368(6487):eaaW5473
Feun LG, Kuo MT, Savaraj N (2015) Arginine deprivation in cancer therapy. Curr Opin Clin Nutr Metab Care 18(1):78–82
Finicle BT, Jayashankar V, Edinger AL (2018) Nutrient scavenging in cancer. Nat Rev Cancer 18(10):619–633
Gaglio D et al (2011) Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 7(1):523
Galluzzi L et al (2013) Metabolic targets for cancer therapy. Nat Rev Drug Discovery 12(11):829–846
Gao X et al (2019) Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572(7769):397–401
Garcia-Bermudez J et al (2018) Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol 20(7):775–781
García-Cañaveras JC, Lahoz A (2021) Tumor microenvironment-derived metabolites: A guide to find new metabolic therapeutic targets and biomarkers. Cancers 13(13):3230
García-Cañaveras JC, Chen L, Rabinowitz JD (2019) The tumor metabolic microenvironment: lessons from lactatethe tumor metabolic microenvironment: lessons from lactate. Can Res 79(13):3155–3162
Gatzka MV (2018) Targeted tumor therapy remixed—an update on the use of small-molecule drugs in combination therapies. Cancers 10(6):155
Geller LT et al (2017) Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357(6356):1156–1160
Gomes C et al (2013) Glycoproteomic analysis of serum from patients with gastric precancerous lesions. J Proteome Res 12(3):1454–1466
Gravel S-P et al (2014) Serine deprivation enhances antineoplastic activity of biguanidesbiguanides and serine depletion. Can Res 74(24):7521–7533
Gregory MA, Nemkov T (2019) Targeting glutamine metabolism and redox state for leukemia therapy. Clin Cancer Res 25(13):4079–4090
Griss T et al (2015) Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol 13(12):e1002309
Gross MI et al (2014) Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 13(4):890–901
Gui DY et al (2016) Environment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. Cell Metab 24(5):716–727
Guile SD et al (2007) Optimization of monocarboxylate transporter 1 blockers through analysis and modulation of atropisomer interconversion properties. J Med Chem 50(2):254–263
Gullapalli S et al (2018) EPL-1410, a novel fused heterocycle based orally active dual inhibitor of IDO1/TDO2, as a potential immune-oncology therapeutic. Cancer Res 78(1701): 10.1158
Hakomori S, Kannagi R (1983) Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst 71(2):231–251
Hao Y et al (2016) Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun 7(1):11971
He Y et al (2021) Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metabol 33(5): 988–1000.e7
Hein AL et al (2016) PR55α subunit of protein phosphatase 2a supports the tumorigenic and metastatic potential of pancreatic cancer cells by sustaining hyperactive oncogenic signaling. Cancer Res 76(8):2243–2253
Hensley CT, Wasti AT, DeBerardinis RJ (2013) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 123(9):3678–3684
Hirata E et al (2015) Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell 27(4):574–588
Huang HY et al (2013) ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res 19(11):2861–2872
Huang Z et al (2018) ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc Natl Acad Sci U S A 115(40):E9499–E9506
Ito K, Suda T (2014) Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15(4):243–256
Julien S et al (2006) ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 16(1):54–64
Kadosh E et al (2020) The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 586(7827):133–138
Kamphorst JJ et al (2015) Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Can Res 75(3):544–553
Kannagi R et al (2008) Current relevance of incomplete synthesis and neo-synthesis for cancer-associated alteration of carbohydrate determinants–Hakomori’s concepts revisited. Biochim Biophys Acta 1780(3):525–531
Kim SM et al (2018) PTEN deficiency and AMPK Activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov 8(7):866–883
Kim SS et al (2020) Histone deacetylase inhibition is synthetically lethal with arginine deprivation in pancreatic cancers with low argininosuccinate synthetase 1 expression. Theranostics 10(2):829–840
Kim H-J, An J, Ha E-M (2022) Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. J Microbiol 60(1):100–117
Kong N, Zhang R, Wu G (2022) Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2112696119
Kraus D et al (2014) Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508(7495):258–262
Kudo T et al (2020) Constitutive expression of the immunosuppressive tryptophan dioxygenase TDO2 in glioblastoma is driven by the transcription factor C/EBPβ. Front Immunol 11:657
Kumar R, Mishra A (2022) Metabolic pathways, enzymes, and metabolites: opportunities in cancer therapy. Cancer 14(21):5268. https://doi.org/10.3390/cancers14215268
Labuschagne CF et al (2014) Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep 7(4):1248–1258
Lambies G, Commisso C (2022) macropinocytosis and cancer: from tumor stress to signaling pathways. Subcell Biochem 98:15–40
Lan Y et al (2020) Codelivered chemotherapeutic doxorubicin via a dual-functional immunostimulatory polymeric prodrug for breast cancer immunochemotherapy. ACS Appl Mater Interface 12(28):31904–31921
Le Floch R et al (2011) CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci 108(40):16663–16668
Le A et al (2010) Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci 107(5):2037–2042
Lee P, Malik D, Perkons N (2020) Targeting glutamine metabolism slows soft tissue sarcoma growth. Nat Commun 11(1):498
Lemberg KM et al (2018) We’re not “DON” Yet: optimal dosing and prodrug delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther 17(9):1824–1832
Leone RD, Zhao L (2019) Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366(6468):1013–1021
Liu Y et al (2012a) A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivoa glut1 inhibitor reduces cancer growth in vitro and in vivo. Mol Cancer Ther 11(8):1672–1682
Liu W et al (2012b) Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci USA 109(23):8983–8988
Liu Y et al (2015) A novel BH 3 mimetic efficiently induces apoptosis in melanoma cells through direct binding to anti-apoptotic Bcl-2 family proteins, including phosphorylated Mcl-1. Pigment Cell Melanoma Res 28(2):161–170
Liu L et al (2018) Inhibition of protein phosphatase 2a sensitizes mucoepidermoid carcinoma to chemotherapy via the PI3K-AKT pathway in response to insulin stimulus. Cell Physiol Biochem 50(1):317–331
Liu H et al (2019) Inflammation-dependent overexpression of c-Myc enhances CRL4(DCAF4) E3 ligase activity and promotes ubiquitination of ST7 in colitis-associated cancer. J Pathol 248(4):464–475
Liu W et al (2022) Covalent organic frameworks as nanocarriers for improved delivery of chemotherapeutic agents. Materials 15(20):7215
Luengo A et al (2021) Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol Cell 81(4):691-707.e6
Lunova M et al (2019) Targeting the mTOR signaling pathway utilizing nanoparticles: a critical overview. Cancer 11(1):82
Ma Y et al (2018) Ovarian cancer relies on glucose transporter 1 to fuel glycolysis and growth: anti-tumor activity of BAY-876. Cancers 11(1):33
Maddocks OD et al (2013) Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493(7433):542–546
Manig F et al (2017) The why and how of amino acid analytics in cancer diagnostics and therapy. J Biotechnol 242:30–54
Marcos NT et al (2004) Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res 64(19):7050–7057
Marcos NT et al (2011) ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front Biosci (elite Ed) 3(4):1443–1455
Marin J et al (2016) Mechanisms of resistance to chemotherapy in gastric cancer. Anti-Cancer Agent Med Chem ACAMC 16(3):318–334
Martinez-Iglesias O et al (2020) Hakin-1, a new specific small-molecule inhibitor for the E3 Ubiquitin-Ligase Hakai, inhibits carcinoma growth and progression. Cancer 12(5):1340
Mashimo T et al (2014) Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159(7):1603–1614
Mei Y et al (2018) RIF1 promotes tumor growth and cancer stem cell-like traits in NSCLC by protein phosphatase 1-mediated activation of Wnt/β-catenin signaling. Cell Death Dis 9(10):942
Meléndez-Rodríguez F et al (2019) HIF1α suppresses tumor cell proliferation through inhibition of aspartate biosynthesis. Cell Rep 26(9):2257-2265.e4
Mendes R et al (2016) Non-small cell lung cancer biomarkers and targeted therapy-two faces of the same coin fostered by nanotechnology. Expert Rev Precis Med Drug Develop 1(2):155–168
Méndez-Lucas A et al (2020) Identifying strategies to target the metabolic flexibility of tumours. Nat Metab 2(4):335–350
Menendez JA et al (2004) Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53–p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int J Oncol 24(3):591–608
Meric-Bernstam F, Gonzalez-Angulo AM (2009) Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27(13):2278–2287
Metallo CM et al (2011) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481(7381):380–384
Miller DM et al (2012) c-Myc and cancer metabolism. Clin Cancer Res 18(20):5546–5553
Miricescu D, Totan A, Stanescu S, Badoiu SC, Stefani C, Greabu M (2020) PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects. Int J Mol Sci 22:173
Miyoshi E, Nakano M (2008) Fucosylated haptoglobin is a novel marker for pancreatic cancer: detailed analyses of oligosaccharide structures. Proteomics 8(16):3257–3262
Mohana-Kumaran N et al (2014) Targeting the intrinsic apoptosis pathway as a strategy for melanoma therapy. Pigment Cell Melanoma Res 27(4):525–539
Molina JR et al (2018) An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med 24(7):1036–1046
Murray CM et al (2005) Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol 1(7):371–376
Nguyen TB et al (2017) DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell 42(1):9-21.e5
Nilsson R et al (2014) Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun 5(1):3128
Nitheesh Y et al (2021) Surface engineered nanocarriers for the management of breast cancer. Mater Sci Eng C Mater Biol Appl 130:112441
Olivares O et al (2017) Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun 8(1):16031
Olson KA, Schell JC, Rutter J (2016) Pyruvate and metabolic flexibility: illuminating a path toward selective cancer therapies. Trends Biochem Sci 41(3):219–230
Oshima N et al (2020) Dynamic imaging of LDH inhibition in tumors reveals rapid in vivo metabolic rewiring and vulnerability to combination therapy. Cell Rep 30(6):1798-1810.e4
Pacold ME et al (2016) A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat Chem Biol 12(6):452–458
Palazzolo S et al (2018) The clinical translation of organic nanomaterials for cancer therapy: a focus on polymeric nanoparticles, micelles liposomes and exosomes. Curr Med Chem 25(34):4224–4268
Park JH, Pyun WY, Park HW (2020) Cancer metabolism: phenotype, signaling and therapeutic targets. Cells 9(10):2308
Paul D et al (2019) Protein phosphatase 1 regulatory subunit sds22 inhibits breast cancer cell tumorigenesis by functioning as a negative regulator of the AKT signaling pathway. Neoplasia 21(1):30–40
Phang JM et al (2012) The proline regulatory axis and cancer. Front Oncol 2:60
Phang JM et al (2015) Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care 18(1):71–77
Pinho SS, Reis CA (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 15(9):540–555
Platten M et al (2014) Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol 5:673
Platten M, Nollen EAA, Röhrig UF (2019) Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 18(5):379–401
Pleguezuelos-Manzano C, et al (2020) Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature. 580(7802): 269–273
Polański R et al (2014) Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in Small cell lung cancerMCT1 inhibition in small cell lung cancer. Clin Cancer Res 20(4):926–937
Possemato R et al (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476(7360):346–350
Qi M et al (2022) The microbiota–gut–brain axis: a novel nutritional therapeutic target for growth retardation. Crit Rev Food Sci Nutr 62(18):4867–4892
Qiu F et al (2014) Arginine starvation impairs mitochondrial respiratory function in ASS1-deficient breast cancer cells. Sci Signal 7(319):ra31
Quanz M et al (2018) Preclinical efficacy of the novel monocarboxylate transporter 1 inhibitor BAY-8002 and associated markers of resistanceanticancer activity of a novel MCT1 inhibitor. Mol Cancer Ther 17(11):2285–2296
Rai G et al (2017) Discovery and optimization of potent, cell-active pyrazole-based inhibitors of lactate dehydrogenase (LDH). J Med Chem 60(22):9184–9204
Reckzeh ES et al (2019) Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth. Cell Chem Biol 26(9):1214-1228.e25
Recouvreux MV, Commisso C (2017) Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front Endocrinol (lausanne) 8:261
Ruan JJ et al (2019) Kidney-type glutaminase inhibitor hexylselen selectively kills cancer cells via a three-pronged mechanism. ACS Pharmacol Transl Sci 2(1):18–30
Sanchez-Macedo N et al (2013) Depletion of the novel p53-target gene carnitine palmitoyltransferase 1C delays tumor growth in the neurofibromatosis type I tumor model. Cell Death Differ 20(4):659–668
Sanderson SM, Gao X (2019) Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer 19(11):625–637
Schug ZT, Vande Voorde J, Gottlieb E (2016) The metabolic fate of acetate in cancer. Nat Rev Cancer 16(11):708–717
Schulte ML et al (2018) Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med 24(2):194–202
Senthebane DA, et al (20200 Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review
Sepich-Poore GD et al (2021) The microbiome and human cancer. Science 371(6536):eabc4552
Shackelford DB et al (2013) LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 23(2):143–158
Singh SK et al (2020) Selective targeting of the hedgehog signaling pathway by PBM nanoparticles in docetaxel-resistant prostate cancer. Cells 9(9):1976
Smith B et al (2016) Addiction to coupling of the warburg effect with glutamine catabolism in cancer cells. Cell Rep 17(3):821–836
Soria J-C et al (2014) Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol 25(11):2244–2251
Sousa CM et al (2016) Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536(7617):479–483
Spinelli JB, Haigis MC (2018) The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 20(7):745–754
Strong AL et al (2015) Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res 17(1):112
Sullivan LB et al (2015) Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162(3):552–563
Sullivan LB et al (2018) Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol 20(7):782–788
Sullivan MR et al (2019) Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab 29(6):1410-1421.e4
Tabish TA, Hamblin MR (2021) Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater Biosyst 3:100023
Tang S et al (2017) Methionine metabolism is essential for SIRT1-regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J 36(21):3175–3193
Tang Y et al (2020a) Selective inhibition of STRN3-containing PP2A phosphatase restores hippo tumor-suppressor activity in gastric cancer. Cancer Cell 38(1):115-128.e9
Tang J et al (2020b) TRIM11 promotes breast cancer cell proliferation by stabilizing estrogen receptor α. Neoplasia 22(9):343–351
Tang L, Mei Y (2021) Nanoparticle-mediated targeted drug delivery to remodel tumor microenvironment for cancer therapy. Int J Nanomed 16:5811–5829
Tennant DA, Durán RV, Gottlieb E (2010) Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10(4):267–277
Thomas D, Rathinavel AK, Radhakrishnan P (2021) Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim Biophys Acta Rev Cancer 1875(1):188464
Valenzuela C et al (2021) Strategies and applications of covalent organic frameworks as promising nanoplatforms in cancer therapy. J Mater Chem B 9(16):3450–3483
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10(9):671–684
Vander Heiden MG, DeBerardinis RJ (2017) Understanding the intersections between metabolism and cancer biology. Cell 168(4):657–669
Walczak K, Turski WA, Rajtar G (2014) Kynurenic acid inhibits colon cancer proliferation in vitro: effects on signaling pathways. Amino Acids 46:2393–2401
Wang Z et al (2019) Methionine is a metabolic dependency of tumor-initiating cells. Nat Med 25(5):825–837
Wang B et al (2022) A randomized phase 3 trial of Gemcitabine or Nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer. Nat Commun 13(1):4025
Ward MP, Spiers JP (2017) Protein phosphatase 2A regulation of markers of extracellular matrix remodelling in hepatocellular carcinoma cells: functional consequences for tumour invasion. British J Pharmacol 174(10):1116–1130
Wei W et al (2020) Cancer registration in China and its role in cancer prevention and control. Lancet Oncol 21(7):e342–e349
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11(1):9–15
Wheaton WW et al (2014) Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 3:e02242
Wieman HL, Wofford JA, Rathmell JC (2007) Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18(4):1437–1446
Wise DR et al (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci 105(48):18782–18787
** H, Kurtoglu M, Lampidis TJ (2014) The wonders of 2-deoxy-d-glucose. IUBMB Life 66(2):110–121
**ang Y et al (2015) Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest 125(6):2293–2306
Xu Y et al (2022) Dual inhibitions on glucose/glutamine metabolisms for nontoxic pancreatic cancer therapy. ACS Appl Mater Interfaces 14(19):21836–21847
Yan H et al (2019) Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett 446:38–48
Yang M, Vousden KH (2016) Serine and one-carbon metabolism in cancer. Nat Rev Cancer 16(10):650–662
Yang L, Venneti S, Nagrath D (2017) Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng 19:163–194
Yang D et al (2017) Huwe1 sustains normal ovarian epithelial cell transformation and tumor growth through the histone H1.3–H19 cascade. Cancer Res 77(18):4773–4784
Yang Q et al (2023) A review of gut microbiota-derived metabolites in tumor progression and cancer therapy. Adv Sci 10(15):2207366
Yeung C et al (2019) Targeting glycolysis through inhibition of lactate dehydrogenase impairs tumor growth in preclinical models of Ewing sarcoma. Can Res 79(19):5060–5073
Yoo HC et al (2020) Glutamine reliance in cell metabolism. Exp Mol Med 52(9):1496–1516
Yoshino H, Enokida H (2020) Characterization of PHGDH expression in bladder cancer: potential targeting therapy with gemcitabine/cisplatin and the contribution of promoter DNA hypomethylation. Mol Oncol 14(9):2190–2202
Yu L et al (2019a) Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells. Cancer Metabol 7:13
Yu S et al (2019b) Advances in nanomedicine for cancer starvation therapy. Theranostics 9(26):8026–8047
Yuneva MO et al (2012) The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab 15(2):157–170
Zabala-Letona A et al (2017) mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature 547(7661):109–113
Zanotelli MR, Zhang J, Reinhart-King CA (2021) Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab 33(7):1307–1321
Zhang L et al (2023) Recent progress in the development of nanomaterials targeting multiple cancer metabolic pathways: a review of mechanistic approaches for cancer treatment. Drug Delivery 30(1):1–18
Zhao S et al (2020) Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol 13(1):1–19
Author information
Authors and Affiliations
Contributions
D.L., Y.W., X.L., Z.W., X.Z. wrote the main manuscript text; D.L., Y.W., Z.Zh., Z.W., X.Z. prepared tables; D.L., Y.W., Z.W., X.Z. prepared figures; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Z. Benfodda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, D., Wang, Y., Li, X. et al. Participation of protein metabolism in cancer progression and its potential targeting for the management of cancer. Amino Acids 55, 1223–1246 (2023). https://doi.org/10.1007/s00726-023-03316-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-023-03316-y