Abstract
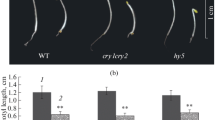
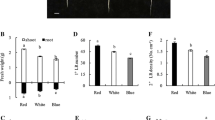
Red light perceived by the shoot bottom suppresses photomorphogenesis in rice seedlings mediated by phytochrome A. Shoots of these seedlings grown in red light having their shoot bottom exposed were deficient in chlorophyll and accumulated high concentration of trans-zeatin riboside. However, reduced presence of isopentynyl adenosine, dihydrozeatin riboside was observed in shoots of red-light-grown non-green seedlings in comparison to green seedling. The message abundance of cytokinin receptor (OsHK5), transporters (OsENT1, OsENT2), and response regulators (OsRR4, OsRR10) was downregulated in these red-light-grown non-green seedlings. Attenuation of greening process was reversed by application of exogenous cytokinin analogue, benzyladenine, or supplementing red light with blue light. In the same vein, the suppression of gene expression of cytokinin receptor, transporters, and type-A response regulators was reversed in red-light-grown seedlings treated with benzyladenine suggesting that the disarrayed cytokinin (CK) signaling cascade is responsible for non-greening of seedlings grown in red light. The reversal of red-light-induced suppression of photomorphogenesis by blue light and benzyladenine demonstrates the interaction of light and cytokinin signaling cascades in the regulation of photomorphogenesis. Partial reversal of greening process by exogenous application of benzyladenine suggests, apart from CKs perception, transportation and responsiveness, other factors are also involved in modulation of suppression of photomorphogenesis by red light.







Similar content being viewed by others
References
Ahmad M, Cashmore AR (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:62–166
Basu D, Dehesh K, Schneider-Poetsch HJ, Harrington SE, McCouch SR, Quail PH (2000) Rice PHYC gene: structure, expression, map position and evolution. Plant Mol Biol 44:27–42
Bilyeu KD, Cole JL, Laskey JG, Riekhof WR, Esparza TJ, Kramer MD, Morris RO (2001) Molecular and biochemical characterization of a cytokinin oxidase from maize. Plant Physiol 125:378–386
Boonman A, Prinsen E, Gilmer F, Schurr U, Peeters AJ, Voesenek LA, Pons TL (2007) Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiol 143:1841–1852
Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A, Okada K, Salomon M, Rudiger W, Sakai T, Takano M, Wada M, Watson JC (2001) The phototropin family of photoreceptors. Plant Cell 13:993–997
Cabrita MA, Baldwin SA, Young JD, Cass CE (2002) Molecular biology and regulation of nucleoside and nucleobase transporter proteins in eukaryotes and prokaryotes. Biochem Cell Biol 80:623–638
Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38:87–117
Cheng X, Jiang H, Zhang J, Qian Y, Zhu S, Cheng B (2010) Overexpression of type-A rice response regulators, OsRR3 and OsRR5, results in lower sensitivity to cytokinins. Genet Mol Res 9:348–359
Choi J, Lee J, Kim K, Cho M, Ryu H, An G, Hwang I (2012) Functional identification of OsHk6 as a homotypic cytokinin receptor in rice with preferential affinity for iP. Plant Cell Physiol 53:1334–1343
Du L, Jiao F, Chu J, ** G, Chen M, Wu P (2007) The two-component signal system in rice (Oryza sativa L.): a genome-wide study of cytokinin signal perception and transduction. Genomics 89:697–707
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, Schäfer E, Fu X, Fan LM, Deng XW (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 45:475–479
Galuszka P, Frebortova J, Werner T, Yamada M, Strnad M, Schmulling T, Frebort I (2004) Cytokinin oxidase/dehydrogenase genes in barley and wheat: cloning and heterologous expression. Eur J Biochem 271:3990–4002
Gupta V, Roy A, Tripathy BC (2010) Signaling events leading to red-light-induced suppression of photomorphogenesis in wheat (Triticum aestivum). Plant Cell Physiol 51:1788–1799
Higuchi M, Pischke MS, Mahonen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, Helariutta Y, Sussman MR, Kakimoto T (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci U S A 101:8821–8826
Hirose N, Makita N, Yamaya T, Sakakibara H (2005) Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiol 138:196–206
Hirose N, Makita N, Kojima M, Kamada-Nobusada T, Sakakibara H (2007) Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol 48:523–539
Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H (2008) Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot 59:75–83
Humayun MZ, Jacob TM (1973) Immunologic studies on nucleic acids and their components. I. An analysis of the specificity of anti-deoxyadenylate antibodies by a membrane-binding technique. Biochim Biophys Acta 331:41–53
Hyde RJ, Cass CE, Young JD, Baldwin SA (2001) The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol 18:53–63
Ito Y, Kurata N (2006) Identification and characterization of cytokinin-signalling gene families in rice. Gene 382:57–65
Iwamoto M, Kiyota S, Hanada A, Yamaguchi S, Takano M (2011) The multiple contributions of phytochromes to the control of internode elongation in rice. Plant Physiol 157:1187–1195
Jain M, Tyagi AK, Khurana JP (2006) Molecular characterization and differential expression of cytokinin-responsive type-A response regulators in rice (Oryza sativa). BMC Plant Biol 6:1
Jumtee K, Okazawa A, Harada K, Fukusaki E, Takano M, Kobayashi A (2009) Comprehensive metabolite profiling of phyAphyBphyC triple mutants to reveal their associated metabolic phenotype in rice leaves. J Biosci Bioeng 108:151–159
Kakimoto T (1996) CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 274:982–985
Kakimoto T (2003) Perception and signal transduction of cytokinins. Annu Rev Plant Biol 54:605–627
Kay SA, Keith B, Shinozaki K, Chye ML, Chua NH (1989) The rice phytochrome gene: structure, auto regulated expression, and binding of GT-1 to a conserved site in the 5′ upstream region. Plant Cell 1:351–360
Kiba T, Aoki K, Sakakibara H, Mizuno T (2004) Arabidopsis response regulator, ARR22, ectopic expression of which results in phenotypes similar to the wol cytokinin-receptor mutant. Plant Cell Physiol 45:1063–1077
Kusnetsov V, Landsberger M, Meurer J, Oelmuller R (1999) The assembly of the CAAT-box binding complex at a photosynthesis gene promoter is regulated by light, cytokinin, and the stage of the plastids. J Biol Chem 274:36009–36014
Li G, Liu K, Baldwin SA, Wang D (2003) Equilibrative nucleoside transporters of Arabidopsis thaliana. cDNA cloning, expression pattern, and analysis of transport activities. J Biol Chem 278:35732–35742
Lubberstedt T, Bolle CE, Sopory S, Flieger K, Herrmann RG, Oelmuller R (1994) Promoters from genes for plastid proteins possess regions with different sensitivities toward red and blue light. Plant Physiol 104:997–1006
Manju RV, Kulkarni MJ, Prasad TG, Sudarshana L, Sashidhar VR (2001) Cytokinin oxidase activity and cytokinin content in roots of sunflower under water stress. Indian J Exp Biol 39:786–792
Mira-Rodado V, Sweere U, Grefen C, Kunkel T, Fejes E, Nagy F, Schafer E, Harter K (2007) Functional cross-talk between two-component and phytochrome B signal transduction in Arabidopsis. J Exp Bot 58:2595–2607
Mok DW, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118
Oh E, Zhu JY, Wang ZY (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14:802–809
Osugi A, Itoh H, Ikeda-Kawakatsu K, Takano M, Izawa T (2011) Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice. Plant Physiol 157:1128–1137
Parks BM, Quail PH, Hangarter RP (1996) Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110:155–162
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta 975:384–394
Quail PH (2002) Photosensory perception and signaling in plant cells: new paradigms? Curr Opin Cell Biol 14:180–188
Rashotte AM, Carson SD, To JP, Kieber JJ (2003) Expression profiling of cytokinin action in Arabidopsis. Plant Physiol 132:1998–2011
Riefler M, Novak O, Strnad M, Schmulling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
Riemann M, Riemann M, Takano M (2008) Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling. Plant Cell Environ 31:783–792
Riemann M, Bouyer D, Hisada A, Müller A, Yatou O, Weiler EW, Takano M, Furuya M, Nick P (2009) Phytochrome A requires jasmonate for photodestruction. Planta 229:1035–1045
Roy A, Sahoo D, Tripathy BC (2013) Involvement of phytochrome A in suppression of photomorphogenesis in rice seedling grown in red light. Plant Cell Environ 36:2120–2134
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449
Shimizu H, Tanabata T, **e X, Inagaki N, Takano M, Shinomura T, Yamamoto KT (2009) Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol Plant 137:289–297
Sood S, Tyagi AK, Tripathy BC (2004) Inhibition of photosystem I and photosystem II in wheat seedlings with their root-shoot transition zones exposed to red light. Photosynth Res 81:31–40
Sood S, Gupta V, Tripathy BC (2005) Photoregulation of the greening process of wheat seedlings grown in red light*. Plant Mol Biol 59:269–287
Suzuki T, Miwa K, Ishikawa K, Yamada H, Aiba H, Mizuno T (2001) The Arabidopsis sensor His-kinase, AHk4, can respond to cytokinins. Plant Cell Physiol 42:107–113
Svyatyna K, Riemann M (2012) Light-dependent regulation of the jasmonate pathway. Protoplasma 249(Suppl 2):S137–S145
Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Baurle I, Kudla J, Nagy F, Schafer E, Harter K (2001) Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294:1108–1111
Takano M, Inagaki N, **e X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, Shinomura T (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of de-etiolation and flowering in rice. Plant Cell 17:3311–3325
Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao Y, Ballaré CL, Sandberg G, Noel JP, Chory J (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176
To JP, Kieber JJ (2008) Cytokinin signaling: two-components and more. Trends Plant Sci 13:85–92
To JP, Haberer G, Ferreira FJ, Deruere J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16:658–671
Tripathy BC, Brown CS (1995) Root-shoot interaction in the greening of wheat seedlings grown under red light. Plant Physiol 107:407–411
Tsai YC, Weir NR, Hill K, Zhang W, Kim HJ, Shiu SH, Schaller GE, Kieber JJ (2012) Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiol 158:1666–1684
Ueguchi C, Sato S, Kato T, Tabata S (2001) The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol 42:751–755
Weiler EW (1980) Radioimmunoassay of tZR and related cytokinins. Planta 149:155–162
Welburn AR, Lichtenthaler H (1984) Formula and program to determine total carotenoids and Chl a and b of leaf extracts in different solvents. In: Sybesma C (ed) Advances in photosynthesis research. Martinus Nijoff/Dr.W. Junk Publishers, The Hague, Vol. II, pp. 9–12
Werner T, Kollmer I, Bartrina I, Holst K, Schmulling T (2006) New insights into the biology of cytokinin degradation. Plant Biol (Stuttg) 8:371–381
Wormit A, Traub M, Florchinger M, Neuhaus HE, Mohlmann T (2004) Characterization of three novel members of the Arabidopsis thaliana equilibrative nucleoside transporter (ENT) family. Biochem J 383:19–26
Yaronskaya E, Vershilovskaya I, Poers Y, Alawady AE, Averina N, Grimm B (2006) Cytokinin effects on tetrapyrrole biosynthesis and photosynthetic activity in barley seedlings. Planta 224:700–709
Acknowledgments
This work was supported by grants from the University Grants Commission, India [No. F. 31-168/2005(SR)], to BCT and PURSE funds obtained from the Department of Science and Technology, Government of India. We acknowledge Dr. V.R. Sashidhar for estimating cytokinin in our samples using antibodies available in his laboratory.
Conflict of interest
There is no conflict of interest for the manuscript entitled “Light-Hormone Interaction in the Red Light Induced Suppression of Photomorphogenesis in Rice Seedlings” submitted to Protoplasma.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 32 kb)
Rights and permissions
About this article
Cite this article
Roy, A., Sahoo, D. & Tripathy, B.C. Light-hormone interaction in the red-light-induced suppression of photomorphogenesis in rice seedlings. Protoplasma 253, 393–402 (2016). https://doi.org/10.1007/s00709-015-0818-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0818-1