Abstract
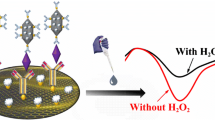
Copper phosphate hybrid nanoflowers (Cu3(PO4)2HNFs) were demonstrated to produce cathodic ECL emission in the presence of potassium persulfate (K2S2O8) and then used as a carrier due to their large specific surface area. AgNPs modified on Cu3(PO4)2HNFs provided more binding sites for immobilizing secondary antibodies and accelerating the electron transfer rate to enhance the ECL signal. In addition, FONDs-Au was used to capture primary antibodies due to its good biocompatibility and large specific surface area. A sandwich electrochemiluminescence (ECL) immunosensor based on copper phosphate hybrid nanoflower/Ag nanoparticle (Cu3(PO4)2HNFs@Ag) composite and Au NPs-functionalized Fe2O3 nanodendrites (FONDs-Au) was constructed to detect prostate-specific antigen (PSA) in real samples. Under optimal conditions, the constructed sandwich ECL immunosensor was sensitive to PSA with a detection limit of 0.037 pg/mL (S/N = 3), a linear detection concentration range of 0.0001–50 ng/mL, and a recovery range of 97.33–102.5%. This immunosensor is expected to provide a method to detect PSA or other biomarkers.
Graphical Abstract







Similar content being viewed by others
References
Najeeb MA, Ahmad Z, Shakoor RA, Mohamed AMA, Kahraman R (2017) A novel classification of prostate specific antigen (PSA) biosensors based on transducing elements. Talanta 168:52–61. https://doi.org/10.1016/j.talanta.2017.03.022
Xu Y, Mu J, Zhou Z, Leng Y, Yu Y, Song X, Liu A, Zhu H, Li J, Wang D (2022) Expansion of mouse castration-resistant intermediate prostate stem cells in vitro. Stem Cell Res Ther 13:299. https://doi.org/10.1186/s13287-022-03207-1
Luo Z, Qin D, Wu Y, Meng S, Mo W, Deng B (2022) An electrochemiluminescence immunosensor based on ABEI-GO-AgNPs as a double-amplified luminophore for the ultra-sensitive detection of prostate-specific antigen. Colloid Surface B 218:112718. https://doi.org/10.1016/j.colsurfb.2022.112718
Zhao Y, Wang R, Xue Y, Jie G (2022) Versatile Au nanoclusters/Au-MnO2 nanoflowers electrochemiluminescence energy transfer platform coupled with rolling circle amplification for dual-targets biosensing of PSA and Let-7a. Sensor Actuat B-Chem 369:132397. https://doi.org/10.1016/j.snb.2022.132397
Dong MM, Lih TSM, Hoti N, Chen SY, Ponce S, Partin A, Zhang H (2021) Development of parallel reaction monitoring assays for the detection of aggressive prostate cancer using urinary glycoproteins. J Proteome Res 20:3590–3599. https://doi.org/10.1021/acs.jproteome.1c00162
Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, Zheng Y, Ye D (2020) Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol 18:282–301. https://doi.org/10.1038/s41585-021-00442-8
Nogueira L, Corradi R, Eastham JA (2009) Prostatic specific antigen for prostate cancer detection. Int Braz J Urol 35:521–531. https://doi.org/10.1590/s1677-55382009000500003
Shen J, Situ B, Du X, Wang Z, Hu R, Li B, Qin AJ, Tang BZ (2022) Aggregation-induced emission luminogen-based dual-mode enzyme-linked immunosorbent assay for ultrasensitive detection of cancer biomarkers in a broad concentration range. ACS Sens 7:766–774. https://doi.org/10.1021/acssensors.1c02237
El-Bayoumy ASA, Keshta ATH, Sallam KM, Ebeid NH, Elsheikh HM, Bayoumy BE (2018) Extraction, purification of prostate-specific antigen (PSA), and establishment of radioimmunoassay system as a diagnostic tool for prostate disorders. J Immunoass Immunoch 39:12–29. https://doi.org/10.1080/15321819.2017.1392320
Zhu T, Tang Q, Zeng Y, Chen S, Yang Y, Wang H, Chen J, Guo L, Li L (2023) Sensitive determination of prostate-specific antigen with graphene quantum dot-based fluorescence aptasensor using few-layer V2CTx MXene as quencher. Spectrochim Acta A 293:122474. https://doi.org/10.1016/j.saa.2023.122474
Wang J, Chen F, Yang Q, Meng Y, Jiang M, Wang Y, Zhang D, Du LP (2023) Light-addressable electrochemical immunoassay for multiplexed detection of antigen. Sensor Actuat B-Chem 374:132821. https://doi.org/10.1016/j.snb.2022.132821
Chong J, Chong H, Lee JH (2019) A chemiluminescent dual-aptasensor capable of simultaneously quantifying prostate specific antigen and vascular endothelial growth factor. Anal Biochem 564:102–107. https://doi.org/10.1016/j.ab.2018.10.024
Lv Z, Zeng R, Zhu L, Qiu Z, Li M, Tang D (2022) Photoelectrochemical immunoassay for prostate-specific antigen based on gasochromic reaction of Pd-MoO3. Sensor Actuat B-Chem 370:132440. https://doi.org/10.1016/j.snb.2022.132440
Liu G, Guan X, Li B, Zhou H, Kong N, Wang H (2022) Hemin-graphene oxide-gold nanoflower-assisted enhanced electrochemiluminescence immunosensor for determination of prostate-specific antigen. Microchim Acta 189:223. https://doi.org/10.1007/s00604-022-05387-2
Yang J, Qin D, Wang N, Wu Y, Fang K, Deng B (2023) Aggregation-induced electrochemiluminescence based on a zinc-based metal–organic framework and a double quencher Au@UiO-66-NH2 for the sensitive detection of amyloid β 42 via resonance energy transfer. Anal Chem 95:7045–7052. https://doi.org/10.1021/acs.analchem.3c00729
Xu L, Hu S, Qin D, Wu Y, Luo Z, Deng B (2023) An electrochemiluminescence immunosensor with double co-reaction accelerators based on Ag3PO4@EuPO4-AgNP for detecting squamous cell carcinoma antigen. Microchim Acta 190:223. https://doi.org/10.1007/s00604-023-05793-0
Zhao L, Song X, Fan D, Liu X, Wang H, Wei Q, Wu D (2023) Highly efficient signal on/off electrochemiluminescence gel aptasensor based on a controlled release strategy for the sensitive detection of prostate specific antigen. Anal Chem 95:5695–5701. https://doi.org/10.1021/acs.analchem.2c05655
Peng L, Li P, Chen J, Deng A, Li J (2023) Recent progress in assembly strategies of nanomaterials-based ultrasensitive electrochemiluminescence biosensors for food safety and disease diagnosis. Talanta 253:123906. https://doi.org/10.1016/j.talanta.2022.123906
Li L, Wang X, Chen J, Huang T, Cao H, Liu X (2023) A novel electrochemiluminescence immunosensor based on resonance energy transfer between g-CN and NU-1000(Zr) for ultrasensitive detection of ochratoxin a in coffee. Foods 12:707. https://doi.org/10.3390/foods12040707
Descamps J, Colin C, Tessier G, Arbault S, Sojic N (2023) Ultrasensitive imaging of cells and sub-cellular entities by electrochemiluminescence. Angew Chem Int Edit 62:e202218574. https://doi.org/10.1002/anie.202218574
Zhang Z, Ma C, Xu Q, Zhu JJ (2022) Recent progress in electrochemiluminescence microscopy analysis of single cells. Analyst 147:2884–2894. https://doi.org/10.1039/d2an00709f
Meng Y, Pu J, Gan J, Li J (2022) Molecularly imprinted electrochemiluminescence sensor based on ZIF-8 doped with CdSe quantum dots for the detection of trace estriol. Luminescence 37:1109–1119. https://doi.org/10.1002/bio.4264
Zhang X, Tian L, Wu K, Sun Z, Wu Q, Shan X, Zhao Y, Chen R, Lu J (2022) High sensitivity electrochemiluminescence sensor based on the synergy of ZIF-7 and CdTe for determination of glucose. Microchem J 177:107254. https://doi.org/10.1016/j.microc.2022.107254
Zhai C, Miao L, Zhang Y, Zhang L, Li H, Zhang S (2022) An enzyme response-regulated colorimetric assay for pattern recognition sensing application using biomimetic inorganic-protein hybrid nanoflowers. Chem Eng J 431:134107. https://doi.org/10.1016/j.cej.2021.134107
Gao J, Liu H, Tong C, Pang L, Feng Y, Zuo M, Wei Z, Li J (2020) Hemoglobin-Mn3(PO4)2 hybrid nanoflower with opulent electroactive centers for high-performance hydrogen peroxide electrochemical biosensor. Sensor Actuat B-Chem 307:127628. https://doi.org/10.1016/j.snb.2019.127628
Ye R, Zhu C, Song Y, Lu Q, Ge X, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) Bioinspired synthesis of all-in-one organic-inorganic hybrid nanoflowers combined with a handheld pH meter for on-site detection of food pathogen. Small 12:3094–3100. https://doi.org/10.1002/smll.201600273
Ge J, Lei J, Zare RN (2012) Protein-inorganic hybrid nanoflowers. Nat Nanotechnol 7:428–432. https://doi.org/10.1038/nnano.2012.80
Yang Y, Liu Y, Pu K, Chen X, Tian H, Gao M, Zhu M, Pan H (2017) Batteries: highly stable cycling of amorphous Li2CO3-Coated α-Fe2O3 nanocrystallines prepared via a new mechanochemical strategy for li-ion batteries. Adv Funct Mater 27:1605011. https://doi.org/10.1002/adfm.201605011
Zhang X, Sui C, Gong J, Su Z, Qu L (2007) Preparation and formation mechanism of different α-Fe2O3 morphologies from snowflake to paired microplates, dumbbell, and spindle microstructures. J Phys Chem C 111:9049–9054. https://doi.org/10.1021/jp0688310
Wang Z, Tu J, Dong P, Bai YN, Han J, **e G (2022) BSA-Cu3(PO4)2 hybrid nanoflowers as a high-performance redox indicator for robust label-free electrochemical immunoassay. Anal Chim Acta 1210:339873. https://doi.org/10.1016/j.aca.2022.339873
Zheng X, Qin D, Hu S, Mo W, Deng B (2023) A sensitive electrochemiluminescence immunosensor for carbohydrate antigen 12–5 analysis based on dual-function SnO2 nanoflowers. Sens Diagn 2:427–437. https://doi.org/10.1039/D2SD00198E
Yang L, Li Y, Zhang Y, Fan D, Pang X, Wei Q, Du B (2017) 3D Nanostructured palladium-functionalized graphene-aerogel-supported Fe3O4 for enhanced Ru(bpy)32+-based electrochemiluminescent immunosensing of prostate specific antigen. ACS Appl Mater Interfaces 9:35260–35267. https://doi.org/10.1021/acsami.7b11458
Shi R, Yang P, Wang J, Zhang A, Zhu Y, Cao Y, Ma Q (2012) Growth of flower-like ZnO via surfactant-free hydrothermal synthesis on ITO substrate at low temperature. CrystEngComm 14:5996–6003. https://doi.org/10.1039/c2ce25606a
Wang J, Chou T, Chen L, Ho KC (2015) Using poly (3-aminophenylboronic acid) thin film with binding-induced ion flux blocking for amperometric detection of hemoglobin A1c. Biosens Bioelectron 63:317–324. https://doi.org/10.1016/j.bios.2014.07.058
He L, Zhang S, Ji H, Wang M, Peng D, Yan F, Fang S, Zhang H, Jia C, Zhang Z (2016) Protein-templated cobaltous phosphate nanocomposites for the highly sensitive and selective detection of platelet-derived growth factor-BB. Biosens Bioelectron 79:553–560. https://doi.org/10.1016/j.bios.2015.12.095
Lee MY, Ding S, Wu C, Peng J, Jiang C, Chou C (2015) Fabrication of nano structured copper phosphate electrodes for the detection of alpha-amino acids. Sensor Actuat B-Chem 206:584–591. https://doi.org/10.1016/j.snb.2014.09.106
Rodriguez-Abetxuko A, Morant-Minana MC, Lopez-Gallego F, Yate L, Seifert A, Knez M, Beloqui A (2018) Imidazole-grafted nanogels for the fabrication of organic-inorganic protein hybrids. Adv Funct Mater 28:1803115. https://doi.org/10.1002/adfm.201803115
Li M, Luo M, Li F, Wang W, Liu K, Liu Q, Wang Y, Lu Z, Wang D (2016) Biomimetic copper-based inorganic-protein nanoflower assembly constructed on the nanoscale fibrous membrane with enhanced stability and durability. J Phys Chem C 120:17348–17356. https://doi.org/10.1021/acs.jpcc.6b03537
Qin D, Meng S, Wu Y, Mo G, Deng B (2022) Aggregation-induced electrochemiluminescence resonance energy transfer with dual quenchers for the sensitive detection of prostate-specific antigen. Sensor Actuat B-Chem 367:132176. https://doi.org/10.1016/j.snb.2022.132176
Funding
The authors received financial support from the National Natural Science Foundation of China (22164004) and the Guangxi Science Foundation of China (2022GXNSFDA035069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Yang, J., Luo, Z. et al. Electrochemiluminescence immunosensor based on Cu3(PO4)2 hybrid nanoflowers as a novel luminophore for the sensitive detection of prostate-specific antigen. Microchim Acta 190, 389 (2023). https://doi.org/10.1007/s00604-023-05966-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05966-x