Abstract
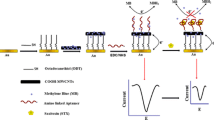
A method is described for single-step detection of V. parahaemolyticus in seafood via aptamer-based SERS. A gold-coated polydimethylsiloxane (PDMS) film was used for the enhancement of Raman scattering. The Raman reporter 4-mercaptobenzoic acid was linked to aptamer modified gold nanoparticles (AuNPs) served as a signalling probe. The negatively charged signalling probe was assembled onto the cysteamine-modified Au-PDMS film through electrostatic adsorption. On addition of V. parahaemolyticus, it will be bound by the aptamer as a biorecognition element, and this leads to the dissociation of the signalling probe from the Au-PDMS film. Hence, the Raman signal (at 1592 cm−1) decreases. The assay has a wide linear response that covers the 1.2 × 102 to 1.2 × 106 cfu·mL−1 V. parahaemolyticus concentration range. The detection limit is 12 cfu·mL−1. The method was successfully applied to the determination of V. parahaemolyticus in oyster and salmon samples.

Schematic presentation of a surface-enhanced Raman spectroscopic method for single step detection of Vibrio parahaemolyticus using gold coated polydimethylsiloxane as the active substrate and aptamer modified gold nanoparticles. This solid substrate simplified the analysis procedures and enhanced the sensitivity.





Similar content being viewed by others
References
Mccarter LL (1999) The multiple identities of Vibrio parahaemolyticus. J Mol Microbiol Biotechnol 1:51–57
Su YC, Liu C (2007) Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 24:549–558
Wang Y, Liu SJ, Pu QK, Li YX, Wang XX, Jiang Y, Yang DN, Yang Y, Yang J, Sun C (2018) Rapid identification of Staphylococcus aureus, Vibrio parahaemolyticus and Shigella sonnei in foods by solid phase microextraction coupled with gas chromatography–mass spectrometry. Food Chem 262:7–13
Wu W, Zhou M, He H, Liu CZ, Li PF, Wang M, Liu Y, Hao X, Fang Z (2018) A sensitive aptasensor for the detection of Vibrio parahaemolyticus. Sensor Actuat B-Chem 272:550–558
Wang Y, Shen X, Gu RR, Shi YF, Tian LL (2015) Application of a rapid method for detecting Vibrio Parahaemolyticus in seafood. J Food Saf 35:26–31
Kumar BK, Raghunath P, Devegowda D, Deekshit VK, Venugopal MN, Karunasagar I (2011) Development of monoclonal antibody based sandwich ELISA for the rapid detection of pathogenic Vibrio parahaemolyticus in seafood. Int J Food Microbiol 145:244–249
Kong C, Wang Y, Fodjo EK, Yang GX, Han F, Shen XS (2018) Loop-mediated isothermal amplification for visual detection of Vibrio parahaemolyticus using gold nanoparticles. Microchim Acta 185:35
Wu SJ, Wang YQ, Duan N, Ma HL, Wang ZP (2015) Colorimetric aptasensor based on enzyme for the detection of Vibrio parahemolyticus. J Agr Food Chem 63:7849–7854
Duan N, Wu S, Zhang H, Zou Y, Wang Z (2018) Fluorometric determination of Vibrio parahaemolyticus using an F0F1-ATPase-based aptamer and labeled chromatophores. Microchim Acta 185:304
Wu SJ, Duan N, Shi Z, Fang CC, Wang ZP (2014) Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal Chem 86:3100–3107
Lane LA, Qian XM, Nie SM (2015) SERS nanoparticles in medicine: from label-free detection to spectroscopic tagging. Chem Rev 115:10489–10529
Lee HK, Lee YH, Phang IY, Wei JQ, Miao YE, Liu TX et al (2014) Plasmonic liquid marbles: a miniature substrate-less SERS platform for quantitative and multiplex ultratrace molecular detection. Angew Chem Int Ed 53:5054–5058
Cao YWC, ** RC, Mirkin CA (2002) Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 297:1536–1540
Sundaram J, Park B, Kwon Y, Lawrence KC (2013) Surface enhanced Raman scattering (SERS) with biopolymer encapsulated silver nanosubstrates for rapid detection of foodborne pathogens. Int J Food Microbiol 167:67–73
Kowalska AA, Kaminska A, Adamkiewicz W, Witkowska E, Tkacz M (2015) Novel highly sensitive Cu-based SERS platforms for biosensing applications. J Raman Spectrosc 46:428–433
Ravindranath SP, Wang YL, Irudayaraj J (2005) SERS driven cross-platform based multiplex pathogen detection. Sensor Actuat B-Chem 152:183–190
Guven B, Basaran-Akgul N, Temur E, Tamer U, Boyaci IH (2011) SERS-based sandwich immunoassay using antibody coated magnetic nanoparticles for Escherichia coli enumeration. Analyst 136:740–748
Driskell JD, Kwarta KM, Lipert RJ, Porter MD, Neill JD, Ridpath JF (2005) Low-level detection of viral pathogens by a surface-enhanced Raman scattering based immunoassay. Anal Chem 77:6147–6154
Duan N, Chang BY, Zhang H, Wang ZP, Wu SJ (2016) Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int J Food Microbiol 218:38–43
Duan N, Shen MF, Wu SJ, Zhao CX, Ma XY, Wang ZP (2017) Graphene oxide wrapped Fe3O4@Au nanostructures as substrates for aptamer-based detection of Vibrio parahaemolyticus by surface-enhanced Raman spectroscopy. Microchim Acta 184:2563–2660
Pan ZH, Wang TC, Sun SF, Zhao BX (2016) Durable microstructured surfaces: combining electrical conductivity with superoleophobicity. ACS Appl Mater Inter 8:1795–1804
Mao HY, Wu WG, She DD, Sun GC, Lv PP, Xu J (2014) Microfluidic surface-enhanced Raman scattering sensors based on nanopillar forests realized by an oxygen-plasma-strip**-of-photoresist technique. Small 10:127–134
Lu G, Li H, Zhang H (2011) Nanoparticle-coated PDMS elastomers for enhancement of Raman scattering. Chem Commun 47:8560–8562
Zhao H, Hasi W, Bao L, Liu YP, Han SQGW, Lin DY (2018) A silver self-assembled monolayer-decorated polydimethylsiloxane flexible substrate for in situ SERS detection of low-abundance molecules. J Raman Spectrosc 49:1469–1477
Qian C, Guo QH, Xu MM, Yuan YX, Yao JL (2015) Improving the SERS detection sensitivity of aromatic molecules by a PDMS-coated Au nanoparticle monolayer film. RSC Adv 5:53306–53312
Duan N, Wu SJ, Chen XJ, Huang YK, Wang ZP (2012) Selection and identification of a DNA aptamer targeted to Vibrio parahaemolyticus. J Agr Food Chem 60:4034–4038
Simpson TRE, Tabatabaian Z, Jeynes C, Parbhoo B, Keddie JL (2004) Influence of interfaces on the rates of crosslinking in poly (dimethyl siloxane) coatings. J Polym Sci Part A: Polym Chem 42:1421–1431
Michota A, Bukowska J (2003) Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J Raman Spectrosc 34:21–25
Acknowledgements
This work was partially supported by the Key Research and Development Program of Jiangsu Province BE2016306, National Key Research and Development Program of China (2018YFC1602905), National Natural Science Fund of China (NSFC 31772086, 31871721), Jiangsu Agriculture Science and Technology Innovation Fund (CX(18)2025) and Young Elite Scientists Sponsorship Program by CAST (2017QNRC001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 828 kb)
Rights and permissions
About this article
Cite this article
Wu, S., Duan, N., Shen, M. et al. Surface-enhanced Raman spectroscopic single step detection of Vibrio parahaemolyticus using gold coated polydimethylsiloxane as the active substrate and aptamer modified gold nanoparticles. Microchim Acta 186, 401 (2019). https://doi.org/10.1007/s00604-019-3499-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3499-1