Abstract
Purpose
To clarify how often postoperative surveillance colonoscopy should be undertaken based on the risk factors for the development of metachronous cancer (MC) and advanced adenoma (AA) after surgery for colorectal cancer.
Methods
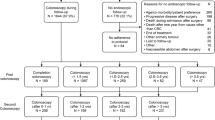
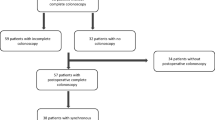
We collected data of consecutive patients who underwent curative resection for primary colorectal cancer between 2005 and 2012, with preoperative colonoscopy and surveillance colonoscopy at 1 year after surgery (406 patients, mean age: 69 years, 59% male). The detection rates of AA (with villous features, > 10 mm or high-grade dysplasia) and MC by surveillance colonoscopy were the primary outcomes.
Results
At 5 years, colonoscopy was performed as postoperative surveillance an average of 3.2 times. AA and MC were detected in 57 (14.0%) and 18 patients (4.4%), respectively. Both lesions were more common in the right colon (n = 43) than in the left colon (n = 28). The detection rate did not differ to a statistically significant extent according to the number of colonoscopies performed for surveillance (p = 0.21). However, after left-sided colectomy, both types of lesions were more commonly detected in those who received ≥ 3 colonoscopies than in those with one or two colonoscopies (p = 0.04).
Conclusion
A remaining right colon after left-sided colectomy was associated with a higher risk of develo** AA and MC. Physicians should consider performing surveillance colonoscopy more frequently if the right colon remains after surgery.




Similar content being viewed by others
References
Togashi K, Konishi F, Ozawa A, Sato T, Shito K, Kashiwagi H, et al. Predictive factors for detecting colorectal carcinomas in surveillance colonoscopy after colorectal cancer surgery. Dis Colon Rectum. 2000;43:S47–53.
Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–99.
Rodriguez-Moranta F, Salo J, Arcusa A, Boadas J, Piñol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–93.
Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–56.
Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. Int J Colorectal Dis. 1997;12:329–34.
Green RJ, Metlay JP, Propert K, Catalano PJ, Macdonald JS, Mayer RJ, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Intern Med. 2002;136:261–9.
Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US multi-society task force on colorectal cancer. Gastroenterology. 2016;150:758-768.e711.
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263.
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi64-72 (Suppl 6).
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–70.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Colon Cancer. Version 2. 2020.
Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1–9.
Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–85.
Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, The National Polyp Study Workgroup, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901–6.
Sano Y, Fujii T, Matsuda T, Oda Y, Kudo S, Igarashi M, et al. Study design and patient recruitment for the Japan Polyp Study. Open Access J Clin Trials. 2014;6:37–44.
Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375–89.
Samadder NJ, Curtin K, Tuohy TM, Pappas L, Boucher K, Provenzale D, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950–60.
Spratt JS Jr, Ackerman LV, Moyer CA. Relationship of polyps of the colon to colonic cancer. Ann Surg. 1958;148:682–96 (discussion 696-688).
Sadahiro S, Ohmura T, Saito T, Suzuki S. Relationship between length and surface area of each segment of the large intestine and the incidence of colorectal cancer. Cancer. 1991;68:84–7.
Matsuda T, Fujii T, Sano Y, Kudo SE, Oda Y, Kaneko K, et al. Randomized comparison of surveillance intervals after colonoscopic removal of adenomatous polyps: results from the Japan Polyp Study. Gastroenterology. 2014;146:S161–2.
Thomas-Gibson S, Rogers P, Cooper S, Man R, Rutter MD, Suzuki N, et al. Judgement of the quality of bowel preparation at screening flexible sigmoidoscopy is associated with variability in adenoma detection rates. Endoscopy. 2006;38:456–60.
Chandran S, Parker F, Vaughan R, Mitchell B, Fanning S, Brown G, et al. Right-sided adenoma detection with retroflexion versus forward-view colonoscopy. Gastrointest Endosc. 2015;81:608–13.
Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75:1197–203.
Hong SN, Sung IK, Kim JH, Choe WH, Kim BK, Ko SY, et al. The Effect of the bowel preparation status on the risk of missing polyp and adenoma during screening colonoscopy: a tandem colonoscopic study. Clin Endosc. 2012;45:404–11.
Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–25.
Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–90.
Payne SR, Church TR, Wandell M, Rösch T, Osborn N, Snover D, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol. 2014;12:1119–26.
Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–6.
Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6.
Minamoto T, Sawaguchi K, Ohta T, Itoh T, Mai M. Superficial-type adenomas and adenocarcinomas of the colon and rectum: a comparative morphological study. Gastroenterology. 1994;106:1436–43.
Sakashita M, Aoyama N, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, et al. Flat-elevated and depressed, subtypes of flat early colorectal cancers, should be distinguished by their pathological features. Int J Colorectal Dis. 2000;15:275–81.
Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81.
Bosman F, Carneiro F, Hruban R, et al. WHO classification of tumours of the digestive system. Lyon (France): IARC Press; 2010.
Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–44.
O’Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–501.
Murakami T, Mitomi H, Yao T, Saito T, Shibuya T, Sakamoto N, et al. Distinct histopathological characteristics in colorectal submucosal invasive carcinoma arising in sessile serrated adenoma/polyp and conventional tubular adenoma. Virchows Arch. 2018;472:383–93.
Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2010;42:1–10.
Rastogi A, Bansal A, Wani S, Callahan P, McGregor DH, Cherian R, et al. Narrow-band imaging colonoscopy—a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc. 2008;67:280–6.
Inoue T, Murano M, Murano N, Kuramoto T, Kawakami K, Abe Y, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol. 2008;43:45–50.
** XF, Chai TH, Shi JW, Yang XC, Sun QY. Meta-analysis for evaluating the accuracy of endoscopy with narrow band imaging in detecting colorectal adenomas. J Gastroenterol Hepatol. 2012;27:882–7.
Nakamura H, Ikematsu H, Osera S, Ito R, Sato D, Minamide T, et al. Visual assessment of colorectal flat and depressed lesions by using narrow band imaging. Endosc Int Open. 2017;05:E1284–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mitsuru Yokota and other co-authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yokota, M., Muto, J., Hashida, K. et al. The necessity of intensive surveillance colonoscopy for patients with a remaining right colon after resection of colorectal cancer: a retrospective cohort study. Surg Today 52, 502–509 (2022). https://doi.org/10.1007/s00595-021-02372-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02372-9