Abstract
Aims
Maternal type 2 diabetes (T2D) can result in adverse pathological outcomes to both the mother and fetus. The present study aimed to investigate the pathological effects of maternal T2D on the gene expression patterns and functions of fetal human umbilical vein endothelial cells (HUVECs), a representative of fetal vascular cells.
Methods
Cell proliferation, apoptosis, mitochondrial ROS production and cell cycle were measured using flowcytometry. Genome-wide expression was measured using Affymetrix microarray. Gene expression of CCND2, STAT1, ITGB8, ALDH2, and ADAMTS5 was measured using real-time PCR.
Results
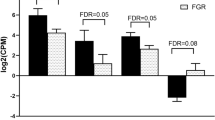
HUVECs derived from T2D mothers (T2D-HUVECs) showed elevated levels of mitochondrial superoxide anions, reduced cell proliferation, and increased apoptosis rates relative to HUVECs derived from healthy control mothers (C.HUVECs). In addition , T2D-HUVECs showed a decreased proportion of cells in G0/G1 and cell cycle arrest at the S phases relative to controls. Interestingly, microarray experiments revealed significant differences in genome-wide expression profiles between T2D-HUVECs and C.HUVECs. In particular, the analysis identified 90 upregulated genes and 42 downregulated genes. The upregulated genes CCND2, STAT1, ITGB8, ALDH2, and ADAMTS5 were validated as potential biomarkers for fetal endothelial dysfunction. Functional network analysis revealed that these genes are the important players that participate in the pathogenesis of endothelial dysfunction, which in turn influences the inflammatory response, cellular movement, and cardiovascular system development and function.
Conclusion
Sustained alterations in the overall function of T2D-HUVEC and gene expression profiles provided insights into the role of maternal T2D on the pathophysiology of the fetal endothelial dysfunction.




Similar content being viewed by others
Abbreviations
- MMPs:
-
ADAMTS are zinc metalloproteases
- AGE:
-
Advanced glycation end products
- ECs:
-
Endothelial cells
- ET-1:
-
Endothelin 1
- ECM:
-
Extracellular matrix
- GDM:
-
Gestational diabetes mellitus
- C.HUVEC:
-
Healthy control cells
- T2D-HUVECs:
-
HUVECs derived from T2D mothers
- T2D:
-
Type 2 diabetes
- NO:
-
Nitric oxide
- PKC:
-
Protein kinase C
- PI:
-
Propidium iodide
- ROS:
-
Reactive oxygen species
References
Vanhoutte PM (2010) Regeneration of the endothelium in vascular injury. Cardiovasc Drugs Ther 24(4):299–303. https://doi.org/10.1007/s10557-010-6257-5
McLaughlin K, Audette MC, Parker JD, Kingdom JC (2018) Mechanisms and clinical significance of endothelial dysfunction in high-risk pregnancies. Can J Cardiol 34(4):371–380. https://doi.org/10.1016/j.cjca.2018.01.006
Zhao Z (2016) Reevaluation of antioxidative strategies for birth defect prevention in diabetic pregnancies. J Biomol Res Ther. https://doi.org/10.4172/2167-7956.1000145
Sultan SA, Liu W, Peng Y, Roberts W, Whitelaw D, Graham AM (2015) The role of maternal gestational diabetes in inducing fetal endothelial dysfunction. J Cell Physiol 230(11):2695–2705. https://doi.org/10.1002/jcp.24993
Petrie JR, Guzik TJ, Touyz RM (2017) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. https://doi.org/10.1016/j.cjca.2017.12.005
Kvietys PR, Granger DN (2012) Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med 52(3):556–592. https://doi.org/10.1016/j.freeradbiomed.2011.11.002
Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I (2006) Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 27(2–3):327–332. https://doi.org/10.1016/j.placenta.2005.01.002
Senthil KKJ, Gokila VM, Wang SY (2017) Activation of Nrf2-mediated anti-oxidant genes by antrodin C prevents hyperglycemia-induced senescence and apoptosis in human endothelial cells. Oncotarget 8(57):96568–96587. https://doi.org/10.18632/oncotarget.19951
Jain M, LoGerfo FW, Guthrie P, Pradhan L (2011) Effect of hyperglycemia and neuropeptides on interleukin-8 expression and angiogenesis in dermal microvascular endothelial cells. J Vasc Surg 53(6):1654–1660. e1652
Zhu ZX, Cai WH, Wang T, Ye HB, Zhu YT, Chi LS, Duan YM, Sun CC, Xuan YH, ** LT (2015) bFGF-regulating MAPKs are involved in high glucose-mediated ROS production and delay of vascular endothelial cell migration. PLoS One 10(12):e0144495. https://doi.org/10.1371/journal.pone.0144495
Barker DJ (1998) In utero programming of chronic disease. Clin Sci (Lond) 95(2):115–128. https://doi.org/10.1042/cs0950115
Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, Knowler WC (2006) Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 55(2):460–465. https://doi.org/10.2337/diabetes.55.02.06.db05-0823
Dabelea D, Knowler WC, Pettitt DJ (2000) Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med 9 (1):83–88. https://doi.org/10.1002/(SICI)1520-6661(200001/02)9:1%3C83::AID-MFM17%3E3.0.CO;2-O
Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda O, Garber AJ, Hirsch IB, Horton ES, Ismail-Beigi F, Jellinger PS, Jones KL, Jovanovic L, Lebovitz H, Levy P, Moghissi ES, Orzeck EA, Vinik AI, Wyne KL, Plan ATFfDaDCC (2011) American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for develo** a diabetes mellitus comprehensive care plan: executive summary. Endocr Pract 17(2):287–302. https://doi.org/10.4158/EP.17.2.287
Eccles KA, Sowden H, Porter KE, Parkin SM, Homer-Vanniasinkam S, Graham AM (2008) Simvastatin alters human endothelial cell adhesion molecule expression and inhibits leukocyte adhesion under flow. Atherosclerosis 200(1):69–79. https://doi.org/10.1016/j.atherosclerosis.2007.12.018
Quintana-Cabrera R, Fernandez-Fernandez S, Bobo-Jimenez V, Escobar J, Sastre J, Almeida A, Bolanos JP (2012) gamma-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat Commun 3:718. https://doi.org/10.1038/ncomms1722
hua Yu J, yu Liu C, bin Zheng G, Zhang LY, hui Yan M, yan Zhang W, ying Meng X, fang Yu X (2013) Pseudolaric acid B induced cell cycle arrest, autophagy and senescence in murine fibrosarcoma l929 cell. Int J Med Sci 10(6):707
Pfaffl MW (2002) Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):36e–36. https://doi.org/10.1093/nar/30.9.e36
Alonso-Calvo R, Maojo V, Billhardt H, Martín-Sánchez F, García-Remesal M, Pérez-Rey D (2007) An agent-and ontology-based system for integrating public gene, protein, and disease databases. J Biomed Inform 40(1):17–29
Shrestha B, Prasai PK, Kaskas AM, Khanna A, Letchuman V, Letchuman S, Alexander JS, Orr AW, Woolard MD, Pattillo CB (2018) Differential arterial and venous endothelial redox responses to oxidative stress. Microcirculation 20:e12486
Patel H, Chen J, Das KC, Kavdia M (2013) Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol 12(1):142. https://doi.org/10.1186/1475-2840-12-142
Villeneuve LM, Natarajan R (2010) The role of epigenetics in the pathology of diabetic complications. Am J Physiol Renal Physiol 299(1):F14–F25. https://doi.org/10.1152/ajprenal.00200.2010
Sank A, Wei D, Reid J, Ertl D, Nimni M, Weaver F, Yellin A, Tuan TL (1994) Human endothelial cells are defective in diabetic vascular disease. J Surg Res 57(6):647–653. https://doi.org/10.1006/jsre.1994.1195
Wang Q, Yang M, Xu H, Yu J (2014) Tetrahydrobiopterin improves endothelial function in cardiovascular disease: a systematic review. Evid Based Complement Altern Med 2014:850312. https://doi.org/10.1155/2014/850312
Su D, Zhou Y, Hu S, Guan L, Shi C, Wang Q, Chen Y, Lu C, Li Q, Ma X (2017) Role of GAB1/PI3K/AKT signaling high glucose-induced cardiomyocyte apoptosis. Biomed Pharmacother 93:1197–1204
Boeynaems S, Tompa P, Van Den Bosch L (2018) Phasing in on the cell cycle. Cell Div 13(1):1. https://doi.org/10.1186/s13008-018-0034-4
Dimauro I, Sgura A, Pittaluga M, Magi F, Fantini C, Mancinelli R, Sgadari A, Fulle S, Caporossi D (2017) Regular exercise participation improves genomic stability in diabetic patients: an exploratory study to analyse telomere length and DNA damage. Sci Rep 7(1):4137. https://doi.org/10.1038/s41598-017-04448-4
Lorenzi M, Cagliero E, Toledo S (1985) Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes 34(7):621–627. https://doi.org/10.2337/diab.34.7.621
Wafa K, MacLean J, Zhang F, Pasumarthi KB (2013) Characterization of growth suppressive functions of a splice variant of cyclin D2. PLoS One 8(1):e53503
Ambra R, Manca S, Palumbo MC, Leoni G, Natarelli L, De Marco A, Consoli A, Pandolfi A, Virgili F (2014) Transcriptome analysis of human primary endothelial cells (HUVEC) from umbilical cords of gestational diabetic mothers reveals candidate sites for an epigenetic modulation of specific gene expression. Genomics 103(5–6):337–348. https://doi.org/10.1016/j.ygeno.2014.03.003
Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, Zou B, Levitt H, Parambil NA, O’Donnell CP (2016) Glucose induces mouse beta cell proliferation via IRS2, mTOR and cyclin D2 but not the insulin receptor. Diabetes 5:db150529
Ando K, Ajchenbaum-Cymbalista F, Griffin JD (1993) Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci USA 90(20):9571–9575. https://doi.org/10.1073/pnas.90.20.9571
Moore F, Naamane N, Colli ML, Bouckenooghe T, Ortis F, Gurzov EN, Igoillo-Esteve M, Mathieu C, Bontempi G, Thykjaer T, Orntoft TF, Eizirik DL (2011) STAT1 is a master regulator of pancreatic {beta}-cell apoptosis and islet inflammation. J Biol Chem 286(2):929–941. https://doi.org/10.1074/jbc.M110.162131
Sikorski K, Czerwoniec A, Bujnicki JM, Wesoly J, Bluyssen HA (2011) STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNgamma, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev 22(4):211–219. https://doi.org/10.1016/j.cytogfr.2011.06.003
Vicente-Manzanares M, Sánchez-Madrid F (2018) Targeting the integrin interactome in human disease. Curr Opin Cell Biol 55:17–23
Abplanalp WT, Conklin DJ, Cantor JM, Ginsberg MH, Wysoczynski M, Bhatnagar A, O’Toole TE (2016) Enhanced integrin α4β1-mediated adhesion contributes to a mobilization defect of endothelial progenitor cells in diabetes. Diabetes 5:db160634
Guo JM, Liu AJ, Zang P, Dong WZ, Ying L, Wang W, Xu P, Song XR, Cai J, Zhang SQ, Duan JL, Mehta JL, Su DF (2013) ALDH2 protects against stroke by clearing 4-HNE. Cell Res 23(7):915–930. https://doi.org/10.1038/cr.2013.69
Zhang Q, Zheng J, Qiu J, Wu X, Xu Y, Shen W, Sun M (2017) ALDH2 restores exhaustive exercise-induced mitochondrial dysfunction in skeletal muscle. Biochem Biophys Res Commun 485(4):753–760
Pan G, Deshpande M, Palaniyandi SS (2017) Decreased aldehyde dehydrogenase (ALDH)2 activity contributes to coronary endothelial dysfunction in diabetic cardiomyopathy. J Mol Cell Cardiol 112:135–136. https://doi.org/10.1016/j.yjmcc.2017.07.019
Takahashi H, Yuge K, Matsubara S, Ohkuchi A, Kuwata T, Usui R, Suzuki M, Takizawa T (2014) Differential expression of ADAM (a disintegrin and metalloproteinase) genes between human first trimester villous and extravillous trophoblast cells. J Nippon Med Sch 81(3):122–129
Akyol S, Ugurcu V, Cakmak O, Altuntas A, Yukselten Y, Akyol O, Sunguroglu A, Demircan K (2016) Evidence for the control of aggrecanases by insulin and glucose in Alzheimer’s Disease. Bull Clin Psychopharmacol 24(4):323–332. https://doi.org/10.5455/bcp.20140905124459
Salomon C, Westermeier F, Puebla C, Arroyo P, Guzman-Gutierrez E, Pardo F, Leiva A, Casanello P, Sobrevia L (2012) Gestational diabetes reduces adenosine transport in human placental microvascular endothelium, an effect reversed by insulin. PLoS One 7(7):e40578
Acknowledgements
The author is grateful for mothers who donated cords and nurses who helped with sample collection in Gynecology and Obstetrics department at King Abdulaziz University Hospital. This work was supported by a grant from King Abdulaziz City for Science and Technology (KACST) Grant no 35-180. The author is also grateful to Dr. H. Schulten, Dr. S. Karim, Dr. F, Ahmed and staff from microarray and Bioinformatics unit at the Center of Excellence in Genomic Medicine Research (CEGMR) for their help and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author declared that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval of the study was given by the Ethics Committee of the King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Porta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sultan, S. The effect of maternal type 2 diabetes on fetal endothelial gene expression and function. Acta Diabetol 56, 73–85 (2019). https://doi.org/10.1007/s00592-018-1207-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1207-y