Abstract
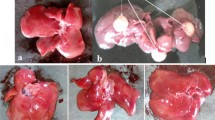
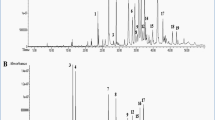
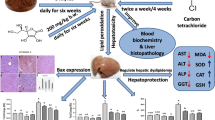
This study evaluated the hepatoprotective effect of oral administration of aqueous fraction of methanolic extract of Costus afer leaves (CALAF) during induction of hepatocellular carcinoma (HCC) with diethylnitrosamine (DEN) in rats. The methanolic leaf extract was fractionated into hexane, ethyl acetate, butanol, and aqueous fractions. The in vitro antioxidant potential of the fractions were estimated by the assays of 2,2-diphenyl-1-picrylhydrazine and nitric oxide radical scavenging activity, ferric-reducing antioxidant potential, and total antioxidant capacity. CALAF had the most antioxidant effect. Rats were orally pretreated daily with CALAF at 100, 200, and 400 mg/kg or silymarin (hepatoprotective drug) at 50 mg/kg from 2 weeks prior to HCC induction and through 6 weeks of HCC induction. The HCC induction was by a single intraperitoneal injection of DEN at 200 mg/kg as an initiator, followed 2 weeks later by daily oral administration of 2-acetylaminofluorene at 30 mg/kg as promoter. At the end of HCC induction, levels of alpha-fetoprotein (AFP), liver function and antioxidants, gamma histone 2A family member X (γH2AX), and O6-methylguanine-DNA methyltransferase (MGMT) expressions were determined. HCC rats treated with CALAF at all doses had significantly (p < 0.05) reduced levels of AFP, aspartate aminotransferase, alanine aminotransferase, superoxide dismutase, glutathione peroxidase, reduced glutathione, and γH2AX protein expression, whereas MGMT protein expression was elevated when compared with untreated HCC rats. Thus, CALAF could be effective in protecting against DEN-induced HCC in rats by ameliorating hepatic injury and genotoxicity.






Similar content being viewed by others
References
Ahmad MS, Suardi N, Shukri A, Mohammad H, Oglat AA, Abunahel BM, Mohamed ABM, Makhamrah O (2019) Current status regarding tumour progression, surveillance, diagnosis, staging, and treatment of hcc: a literature review. J Gastroenterol Hepatol Res 8:2841–2852
Amereh Z, Hatami N, Shirazi F, Gholami S, Hosseini HS, Noubarani M, Kamalinejad M, Andalib S, Keyhanfar F, Eskandari RM (2017) Cancer chemoprevention by oleaster (Elaeagnus angustifoli L.) fruit extract in a model of hepatocellular carcinoma induced by diethylnitrosamine in rats. ExCLI J Sci 16:1046–1056
Anaga AO, Njoku CJ, Ekejuiba ES, Esiaka MN, Asuzu IU (2004) Investigations of the methanolic leaf extract of Costus afer Ker Gawl. for pharmacological activities in vitro and in vivo. Phytomed 11:242–248
Anyakeme T, Essien ES, Akinnawor JO (2014) Hepatoprotective effect of methanolic stem extract of bush cane (Costus afer) on immunologic response generated reactive oxygen species (ROS) in alcohol-induced liver cirrhosis in rats. Asian J Biochem Pharm Res 4:215–223
Anyasor GN, Onajobi F, Osilesi O, Adebawo O (2014) Proximate composition, mineral content and in vitro antioxidant activity of leaf and stem of Costus afer (ginger lily). J Intercult Ethnopharmacol 3:128–134
Anyasor GN, Onajobi F, Osilesi O, Adebawo O, Oboutor E (2015) Evaluation of Costus afer Ker Gawl. in vitro anti-inflammatory activity and its chemical constituents identified using gas chromatography mass spectrometry analysis. J Coast Life Med 3:132–138
Aweke G (2007) Costus afer Ker Gawl. record from PROTA4U. Schmelzer GH & Gurib-Fakim A (Editors). PROTA (Plant Resources of Tropical Africa/Ressourcesvégétales de l’Afrique Tropicale), Wageningen, Netherlands. Retrieved December 16, 2019, from http://www.prota4u.org/search.asp.
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Ann Biochem 239:70–76
Biselli M, Conti F, Gramenzi A, Frigerio M, Cucchetti A, Fatti G, D’Angelo M, Dall’Agata M, Giannini EG, Farinati F, Ciccarese F, Andreone P, Bernardi M, Trevisani F (2015) A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br J Cancer 112:69–76
Boison D, Adinortey CA, Babanyinah GW, Quasie O, Agbeko R, Wiabo-Asabil GK, Adinortey MB (2019) Costus afer: a systematic review of evidence-based data in support of its medicinal relevance. Scientifica (Cairo) 2019:3732687–3732610. https://doi.org/10.1155/2019/3732687
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:1–31. https://doi.org/10.3322/caac.21492
Buetler E, Duran O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Castro-López C, Bautista-Hernández I, González-Hernández MD, Martínez-Ávila GCG, Rojas R, Gutiérrez Díez A, Medina-Herrera N, Aguirre-Arzola VE (2019) Polyphenolic profile and antioxidant activity of leaf purified hydroalcoholic extracts from seven Mexican Persea americana cultivars. Molecules 24(1):173. https://doi.org/10.3390/molecules24010173
Chedid F, Kruel C, Pinto M, Grezzan-filho T, Ian L (2017) Hepatocellular carcinoma: diagnosis and operative management. Arq Bras Cir Dig 30:272–278
Chen S, Liu H, Chen S, Lin P, Lai C et al (2017) Changes of oxidative stress, glutathione and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS One 12:e0170016. https://doi.org/10.1371/journal.pone.0170016
Chou W-C, Lee C-N, Yang T-S, Huang C-Y, Teng W, Tseng Y-T, Chen J-S, Lin Y-C, Hou M-M, Chang HH, Hsieh JC-H (2018) Changes in serum α-fetoprotein level predicts treatment response and survival in hepatocellular carcinoma patients and literature review. J Formos Med Assoc 117(2):153–163
Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR (2010) Antioxidant and free radical scavenging activity of H. officinalis, L. Var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci 23:29–34
El-Far AH, Badria FA, Shaheen HM (2016) Possible anticancer mechanisms of some Costus speciosus active ingredients concerning drug discovery. Curr Drug Discov Technol 13(3):123–143
Ezejiofor AN, Orisakwe OE (2017) Evaluation of protective effect of aqueous leave extract of Costus afer on female albino Wistar rats exposed to lead acetate. EC Pharmacol Toxicol 4:75–92
Ezejiofor AN, Orish CN, Orisakwe OE (2013) Effect of aqueous leaves extract of Costus afer Ker Gawl. (Zingiberaceae) on the liver and kidney of male albino Wistar rat. Anc Sci Life 33:4–9
Flohe L, Gunzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/s0076-6879(84)05015-1
Fujiwara N, Friedman S, Goossens N, Hoshida Y (2017) Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol 68:526–549
Hassan HA, El-Gharib NE, Azhari AF (2016) Role of natural antioxidants in the therapeutic management of hepatocellular carcinoma. Hepatom 2:216–223. https://doi.org/10.20517/2394-5079.2016.12
Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennet WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC (2000) Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res 60:3333–3337
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidant maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44:532–553. https://doi.org/10.1159/000485089
Huang ZZ, Chen C, Zeng Z, Yang H, Oh H et al (2010) Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver generation. FASEB 15:19–21
Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Olga A, Martin OA (2012) Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 321(1–2):123–133. https://doi.org/10.1016/j.canlet.2011.12.025
Jacinto FV, Esteller M (2007) Mutator pathways unleashed by epigenetic silencing in human cancer. Mutagenesis 22:247–253
Jahan MS, Vani G, Shyamaladevi CS (2011) Anticarcinogenic effect of Solarium trilobatum in diethylnitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rats. Asian J Biochem 6(1):74–81
Kaina B, Fahrer J (2013) O6-methylguanine-DNA methyltransferase in the defense against N-nitroso compounds and colorectal cancer. Carcinogen 34(11):2435–2442. https://doi.org/10.1093/carcin/bgt275
Kaina B, Christmann M, Naumann S, Roos WP (2007) Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 6:1079–1099
Li F, Fernandez PP, Rajendra P, Hui KM, Sethi G (2010) Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett 292:197–207
Lin RC, Hanquet B, Mareh-Aleth L (1996) Aferoside A, a steroidal saponin from Costus afer. Phytochem 43:665–668
Lin RC, Lacaille-Dubois M-A, Hanquet B, Correia M, Bruno C (1997) New diosgenin glycosides from Costus afer. J Nat Prod 60(11):1165–1169. https://doi.org/10.1021/np9702190
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G (2016) Hepatocellular carcinoma. Nat Rev Dis Primers 2:16018. https://doi.org/10.1038/nrdp.2016.18
Lourenço SC, Moldão-Martins M, Alves VD (2019) Antioxidants of natural plant origins: from sources to food industry applications. Molecules 24:4132. https://doi.org/10.3390/molecules24224132
Marengo B, Ciucis CD, Ricciarelli R, Romano P, Passalacquaet M et al (2010) DNA oxidative damage of neoplastic rat liver lesions. Oncol 23:1241–1246
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzyme function for erythrocuprein. J Biol Chem 244:6049–6055
McCune LM, Johns T (2002) Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmcol 82:197–205
Mittal S, El-Serag HB (2013) Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 47:2–6
Nair SVG, Hettihewa M, Rupasinghe HP (2014) Apoptotic and inhibitory effects on cell proliferation of hepatocellular carcinoma HepG2 cells by methanol leaf extract of Costus speciosus. Biomed Res Int 2014. https://doi.org/10.1155/2014/637098
National Institutes of Health (2011) Guide for the Care and Use of Laboratory Animals, 8th edn. The National Academies Press, Washington DC
Organization for Economic Cooperation Development (2001) The OECD Guideline for Testing of Chemical. The Organization of Economic Co-operation Development, Paris
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243
Park SJ, Jan JY, Jeong SW, Cho YK, Lee SH et al (2017) Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 96:e5811. https://doi.org/10.1097/MD.0000000000005811
Prashant T, Bimlesh K, Mandeep K, Gurpreet K, Harleen K (2011) Phytochemical screening and extraction: a review. Int Pharm Sci 1:98–106
Prieto RL, Pineda M, Aquilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Ann Biochem 269:337–341
Salvatore R, Giuseppe F (2018) Role, targets and regulation of (de)nitrosylation in malignancy. Front Oncol 8:334. https://doi.org/10.3389/fonc.2018.00334
Sarikaya E, Dogan S (2020) Glutathione peroxidase in health and disease. Intechopen. https://doi.org/10.5772/intechopen.91009
Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, Yano M, Ishikawa O, Imaoka S (2006) Risk of recurrence in a long term follow-up after surgery in 417 patients with hepatitis B or hepatitis C related hepatocellular carcinoma. Ann Surg 244:771–780
Selim S, Al-Jaouni S (2015) Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Complement Altern Med 15:301. https://doi.org/10.1186/s12906-0150836-8
Sethi G, Shanmugan MK, Warrier S, Merarchi M, Arfuso F et al (2018) Pro-apoptotic and anti-cancer properties of diogenin: a comprehensive and critical review. Nutrients 10:645. https://doi.org/10.3390/nu100050645
Shiraishi A, Sakumi K, Sekihuchi M (2000) Increased susceptibility to chemotherapeutic alkylating agents of mice deficient in DNA repair methyltransferase. Carcinogenesis 21:1879–1883
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA (2019) ROS generation and antioxidant defense systems in normal and malignant cells. Ox Med Cell Lonev 2019 6175804 https://doi.org/10.1155/2019/61758046175817.
Soll JM, Sobol RW, Mosammaparast N (2016) Regulation of DNA alkylation damage repair: lessons and therapeutic opportunities. Trends Biochem Sci 42(3):206–218. https://doi.org/10.1016/j.tibs.2016.10.001
Su XY, Zhao JQ, Li N, Kumar M, Yang AU (2019) Chemoprotective effects of resveratrol against diethylnitrosamine induced hepatocellular carcinoma in wistar rats. Int J Pharmacol 15:549–559. https://doi.org/10.3923/ijp.2019.549.559
Sudan R, Bhagat M, Gupta S, Singh J, Koul A (2014) Iron chelation, ferric reducing antioxidant power and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). Biomed Res Int 2014:179865–179867. https://doi.org/10.1155/2014/179865
Tcheghebe OT, Tala VR, Fouodjouo M (2018) Ethnobotanical uses, phytochemical and pharmacological profiles and toxicity of Costus afer ker Gawl. J Sci Res Allied Sci 4:1–11
Tolba R, Kraus T, Liedtke C, Schwarz M, Weiskirchen R (2015) Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab Anim 49:59–69. https://doi.org/10.1177/0023677215570086
Umoh IU, Aquaisua AN, Udo NM (2019) The effect of fresh stem juice extract of Costus afer on the cytohistomorphology of the kidney in aspirin-treated wistar rats. J Appl Biol Biotechnol 7:78–81. https://doi.org/10.7324/JABB.2019.70214
Vairappan B (2015) Endothelial dysfunction in cirrhosis: role of inflammation and oxidative stress. World J Hepatol 7(3):443–459. https://doi.org/10.4254/wjh.v7.i3.443
Vargas-Mendoza N, Madrigal-Santillan E, Morales-Gonzalez A, Esquivel-Soto J, Esquivel-Chirino C, Garcia Luna M, Gonzalez-Rubio MG, Gayosso-de-Lucio JA, Morales-Gonzalez JA (2014) Hepatoprotective effect of silymarin. World J Hepatol 6:144–149
Veeraraghavan VP, Mohan SK, Jainu M, Seshadri C (2015) Ameliorating effects of Garcinia mangostana Linn pericarp extract on hepatic antioxidants in diethylnitrosamine (DEN) induced hepatocellular carcinoma (HCC). Indian J Pharm Educ Res 49:344–352
Verna L, Whysner J, Williams GM (1996) N-Nitrodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 71:57–81
Xu JH, Chang WH, Fu HW, Yuan T, Chen P (2018) The mRNA, miRNA and lncRNA networks in hepatocellular carcinoma: an integrative transcriptomic analysis from gene expression omnibus. Mol Med Rep 17:6472–6482
Acknowledgments
The authors express deep gratitude to the Director, Research Innovation and International Cooperation, Babcock University with grant number BU/RIIC/2018/008. We appreciate the Department of Biochemistry, Babcock University for providing support and research facility that enabled the completion of this study. We thank Mrs. Chiamaka Anyasor for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial and nonfinancial competing interest concerning this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anyasor, G.N., Idowu, D.P. & Nabofa, W. Evaluation of the hepatoprotective effect of oral administration of aqueous fraction of methanolic extract of Costus afer leaves during induction of hepatocellular carcinoma with diethylnitrosamine in rats. Comp Clin Pathol 29, 733–744 (2020). https://doi.org/10.1007/s00580-020-03124-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-020-03124-w