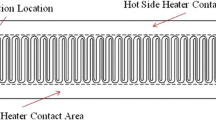
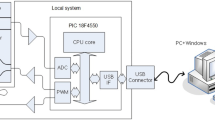
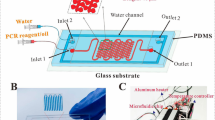
Abstract
Infectious diseases are illnesses caused by harmful pathogens from the exterior. Vaccines could prevent many people from disease infection. Over the past several years, the development and application of molecular diagnostic techniques have launched a revolution in monitoring infectious diseases. Polymerase chain reaction (PCR)-based systems to diagnose the etiologic agents of disease from clinical samples have been applicable in pathogen detection. We demonstrate a microfluidic chip for continuous flow PCR. The PDMS/glass bonding chip provides a miniaturized, cheap, and disposable material for pathogen diagnosis. The homemade thermal control module integrated with two cartridge heaters and one Peltier element supports the denaturation, extension, and annealing regions created inside the chip. Due to the large surface-to-volume ratio in the microchannel, the surface characteristics might augment the protein adsorption onto the channel surfaces and reduce the PCR amplification efficiency. We measure the hydrophilic properties, roughness of the wall and the structure of the surface under various surface treatments and express the PCR amplification efficiency. Results show that the most detailed is the PDMS sheet with the modification of Tween 20 of a concentration of 20% for the reduction of methyl peak of the absorption spectrum, the lowest contact angle, and the minor surface roughness. Next, the continuous flow PCR system amplifies a 385-bp segment of Q fever virus DNA to evaluate the performance of the DNA amplification. The overall product of Tween 20 is good because Tween 20 is an emulsifier, and it has excellent performance in both adhesion properties and coated samples. The need for point-of-care test (PoCT) devices has increased rapidly since the outbreak of COVID-19. The current portable device for PoCT will provide essential tools for real-time diagnosis.
















Similar content being viewed by others
References
Cao Q, Mahalanabis M, Chang J, Carey B, Hsieh C, Stanley A, Odell CA, Mitchell P, Feldman J, Pollock NR, Klapperich CM (2012) Microfluidic Chip for Molecular Amplification of Influenza A RNA in human respiratory specimens. PLoS ONE 7(3):e33176
Chen L, West J, Auroux PA, Manz A, Day PJ (2007) Ultrasensitive PCR and real-time detection from human genomic samples using a bidirectional Flow Microreactor. Anal Chem 79(23):9185–9190
Chen JJ, Shen CM, Ko YW (2013) Analytical study of a microfludic DNA amplification chip using Water cooling Effect. Biomed Microdevices 15(2):261–278
Chen JJ, Liao MH, Li KT, Shen CM (2015) One-heater Flow-through polymerase chain reaction device by heat pipes cooling. Biomicrofluidics 9(1):014107
Chiou CH, Shin DJ, Zhang Y, Wang TH (2013) Topography-assisted electromagnetic platform for blood-to-PCR in a Droplet. Biosens Bioelectron 50:91–99
Coutlée F, Voyer H (1998) Effect of nonionic detergents on amplification of human papillomavirus DNA with consensus primers MY09 and MY11. J Clin Microbiol 36(4):1164
Erickson D, Liu X, Venditti R, Li D, Krull UJ (2005) Electrokinetically based approach for single-nucleotide polymorphism discrimination using a microfluidic device. Anal Chem 77(13):4000–4007
Felbel J, Bieber I, Pipper J, Köhler JM (2004) Investigations on the compatibility of chemically oxidized Silicon (SiOx)-Surfaces for applications towards chip-based polymerase chain reaction. Chem Eng J 101(1–3):333–338
Felbel J, Reichert A, Kielpinski M, Urban M, Henkel T, Häfner N, Dürst M, Weber J (2008) Reverse transcription-polymerase chain reaction (RT-PCR) in Flow-through Micro-reactors: thermal and fluidic concepts. Chem Eng J 135:S298–S302
Fukuba T, Yamamoto T, Naganuma T, Fujii T (2004) Microfabricated Flow-through device for DNA amplification—towards in Situ Gene Analysis. Chem Eng J 101(1–3):151–156
Han D, Jang YC, Oh SN, Chand R, Lim KT, Kim KI, Kim YS (2014) MCU Based Real-Time Temperature Control System for Universal Microfluidic PCR Chip. Microsyst Technol 20:471–476
Hsieh HY, Chang R, Huang YY, Juan PH, Tahara H, Lee KY, Vo DNK, Tsai MH, Wei PK, Sheen HJ (2022) Fan, Continuous Polymerase Chain Reaction Microfluidics Integrated with a gold-capped Nanoslit sensing chip for Epstein-Barr Virus Detection. Biosens Bioelectron 195:113672
Jiang X, Shao N, **g W, Tao S, Liu S, Sui G (2014) Microfluidic chip integrating high Throughput continuous-Flow PCR and DNA hybridization for Bacteria analysis. Talanta 122:246–250
Johnson LM, Gao L, Shields IV CW, Smith M, Efimenko K, Cushing K, Genzer J, López GP (2013) Elastomeric microparticles for acoustic mediated bioseparations. J Nanobiotechnol 11:1–8
Joung SR, Kang CJ, Kim YS (2008) Series DNA amplification using the continuous-Flow polymerase chain reaction chip. Jpn J Appl Phys 47(2S):1342
Kaprou GD, Papadopoulos V, Papageorgiou DP, Kefala I, Papadakis G, Gizeli E, Chatzandroulis S, Kokkoris G, Tserepi A (2019) Ultrafast, Low-Power, PCB Manufacturable, continuous-Flow Microdevice for DNA amplification. Anal Bioanal Chem 411:5297–5307
Kim JA, Lee JY, Seong S, Cha SH, Lee SH, Kim JJ, Park TH (2006) Fabrication and characterization of a PDMS–Glass hybrid continuous-Flow PCR chip. Biochem Eng J 29(1–2):91–97
Kim HE, Schuck A, Kim WY, Jung EK, Hong YH, Kim YS (2022) PID temperature control system-based microfluidic PCR chip for genetic analysis. J Electr Eng Technol 17:495–501
Kopparthy VL, Crews ND (2020) Oscillating-Flow Microfluidic PCR system utilizing a thermal gradient for nucleic acid analysis. Biotechnol Bioeng 117(5):1525–1532
Kramer MF, Coen DM (2001) Enzymatic amplification of DNA by PCR: standard procedures and optimization. Curr Protoc Mol Bio 56(1):15–11
Kuan I, Gu W, Wu J, Wei C, Chen K, Yu C (2008) Effects of Grafting Poly (Ethylene Oxide) on the amplification efficiency of a poly (Dimethylsiloxane)-Based Flow-through PCR device. Chem Eng J 143(1–3):326–330
Kulkarni MB, Goyal S, Dhar A, Sriram D, Goel S (2021) Miniaturized and IoT enabled continuous-Flow-based microfluidic PCR device for DNA amplification. IEEE Trans Nanobiosci 21(1):97–104
Kwak BJ, Kim H, Park N, Hahn JH (2021) Microchip for continuous DNA analysis based on gel Electrophoresis coupled with co-injection of size markers and in-Channel staining. Anal Bioanal Chem 413(23):5685–5694
Li S, Fozdar DY, Ali MF, Li H, Shao D, Vykoukal DM, Floriano PN, Olsen M, McDevitt JT, Gascoyne PRC, Chen S (2006) A continuous-Flow polymerase chain reaction microchip with Regional Velocity Control. J Microelectromechanical Syst 15(1):223–236
Mullis KB, Faloona FA (1987) Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods in Enzymology, vol 155. Academic, pp 335–350
Nakayama T, Kurosawa Y, Furui S, Kerman K, Kobayashi M, Rao SR, Yonezawa Y, Nakano K, Hino A, Yamamura S, Takamura Y, Tamiya E (2006) Circumventing air bubbles in Microfluidic systems and quantitative continuous-Flow PCR applications. Anal Bioanal Chem 386(5):1327–1333
Peham JR, Grienauer W, Steiner H, Heer R, Vellekoop MJ, Nöhammer C (2011) Wiesinger-Mayr, Long Target Droplet polymerase chain reaction with a Microfluidic device for high-throughput detection of pathogenic Bacteria at clinical sensitivity. Biomed Microdevices 13:463–473
Qin K, Lv X, **ng Q, Li R, Deng Y (2016) A BSA coated NOA81 PCR chip for gene amplification. Anal Methods 8(12):2584–2591
Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of Sickle Cell Anemia. Science 230(4732):1350–1354
Schneegaß I, Bräutigam R, Köhler JM (2001) Miniaturized Flow-through PCR with different template types in a Silicon Chip Thermocycler. Lab Chip 1(1):42–49
Seetasang S, Xu Y (2022) Recent progress and perspectives in applications of 2-Methacryloyloxyethyl phosphorylcholine polymers in Biodevices at Small scales. J Mater Chem B 10:2323–2337
Trinh KTL, Lee NY (2022) Fabrication of wearable PDMS device for rapid detection of nucleic acids via recombinase polymerase amplification operated by human body heat. Biosensors 12(2):72
Wang JH, Chien LJ, Hsieh TM, Luo CH, Chou WP, Chen PH, Chen PJ, Lee DS, Lee GB (2009) A miniaturized quanti-tative polymerase chain reaction system for DNA amplification and detection. Sens. Actuators B Chem 141(1):329–337
Wu J, Guo W, Wang C, Yu K, Ma Y, Chen T, Li Y (2015) Research to improve the efficiency of double stereo PCR microfluidic chip by passivating the Inner Surface of Steel Capillary with NOA61. Cell Biochem Biophys 72:605–610
**a YM, Hua ZS, Srivannavit O, Ozel AB, Gulari E (2007) Minimizing the Surface Effect of PDMS–Glass microchip on polymerase chain reaction by dynamic polymer passivation. J Chem Technol Biotechnol 82(1):33–38
Acknowledgements
The authors thank the National Science and Technology Council of the Republic of China for financially supporting this research under Contract No. MOST 110-2313-B-020-003-.
Author information
Authors and Affiliations
Contributions
J.J.C. and X.C.Q. conceptualized the design, and X.C.Q. implemented the platform. J.J.C. supervised the project. J.J.C. and X.C.Q. prepared the initial draft. J.J.C. got the funding. All the authors contributed to the manuscript finalization.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J.J., Qiu, X.C. The effect of the surface passivation on polymerase chain reaction inside a continuous flow microfluidic chip. Microsyst Technol (2024). https://doi.org/10.1007/s00542-024-05675-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00542-024-05675-2