Abstract
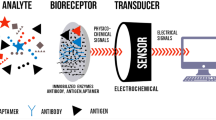
Biosensor is an analytical device to detect the biomolecules assisted by the transducer and physicochemical detector. A good biosensor is expecting to be with low cost, easy to perform and identify the results without prior experience. In addition, a good biosensor has two main key characteristics such as sensitivity and specificity; these are mainly determined by the affinity of biomolecules with the assistance of sensing system. Microfluidic-based lab-on-chip is one of the fast growing technologies in the field of biosensor bring the positive characteristics with a fast delivery set-up. On the other hand, gold nanoparticle (GNP) is the powerful tool to enhance the biomolecular detection with higher sensitivity and it has been proved for the effective applications with different sensors. In this review, we discussed the applications of microfluidic-based delivery and GNP for biosensing with the new level of developments, which elevate a step ahead.





Similar content being viewed by others
References
Ashok P, Dholakia K (2012) Microfluidic Raman spectroscopy for bio-chemical sensing and analysis. In: Fritzsche W, Popp J (eds) Optical Nano- and Microsystems for Bioanalytics. Springer series on chemical sensors and biosensors, vol. 10, pp 247–268
Balakrishnan SR, Hashim U, Gopinath SCB, Poopalan P, Ramayya HR, Veeradasan P, Haarindraprasad R, Ruslinda AR (2016) Polysilicon nanogap lab-on-chip facilitates multiplex analyses with single analyte. Biosens Bioelectron 84:44–52
Boca S, Rugina D, Pintea A, Leopold N, Astilean S (2012) Designing gold nanoparticle-ensembles as surface enhanced raman scattering tags inside human retinal cells. J Nanotechnol 2012:961216. doi:10.1155/2012/961216
Bosco FG, Yang J, Chen CH, Hwu E-T, Keller SS, Bache M, Lin Q, Boisen A (2012) Micromechanical aptasensor-based protein detection using a compact-disc format microfluidics system. In: Proc. IEEE Int. Conf. Micro Electro Mech. Syst, pp 858–861
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R (1994) Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid? Liquid system. J Chem Soc Chem Commun 7:801–802
Chen C (2014) Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal Chem 86:6843–6849
Chen KN, Liu H, Wang SM, Zheng YJ, Zhu C, Wang Y, Zhu SN (2011) Coherent magnetic plasmon modes in a contacting gold nano-sphere chain on a gold slab. Opt Express 19:23782–23789
Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK (2011) Microfluidics-based diagnostics of infectious diseases in the develo** world. Nat Med 17:1015–1019
de la Rica R, Stevens MM (2012) Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol 8:1759–1764
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (2010) Selective colorimetric detection of polynucleotides properties of gold nanoparticles selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1078:1078–1081
Eteshola E, Balberg M (2004) Microfluidic ELISA: on-chip fluorescence imaging. Biomed Microdevices 6:7–9
Fujii T (2002) PDMS-based microfluidic devices for biomedical applications. Microelectron Eng 61–62:907–914
Fujimaki M, Nomura K, Sato K, Kato T, Gopinath SCB, Wang X, Awazu K, Ohki Y (2010) Detection of colored nanomaterials using evanescent field-based waveguide sensors. Opt Express 18:15732–15740
Gopinath SCB (2010) Biosensing applications of surface plasmon resonance-based Biacore technology. Sens Actuators B Chem 150:722–733
Gopinath SCB, Kumar PKR (2013) Aptamers that bind to the hemagglutinin of the recent pandemic influenza virus H1N1 and efficiently inhibit agglutination. Acta Biomater 9:8932–8941. doi:10.1016/j.actbio.2013.06.016
Gopinath SCB, Shikamoto Y, Mizuno H, Kumar PKR (2007) Snake venom-derived factor IX binding protein specifically blocks the Gla domain-mediated-membrane binding of human factors IX and X. Biochem J 405:351–357
Gopinath SCB, Awazu K, Kumar PKR, Tominaga J (2008) Monitoring biomolecular interactions on a digital versatile disc: a BioDVD platform technology. ACS Nano 2:1885–1895
Gopinath SCB, Awazu K, Fujimaki M, Sugimoto K, Ohki Y, Komatsubara T, Tominaga J, Kumar PKR (2009) Monitoring surface-assisted biomolecular assembly by means of evanescent-field-coupled waveguide-mode nanobiosensors. Anal Bioanal Chem 394:481–488
Gopinath SCB, Kumaresan R, Awazu K, Fujimaki M, Mizuhata M, Tominaga J, Kumar PKR (2010) Evaluation of nucleic acid duplex formation on gold over layers on biosensor fabricated using Czochralski-grown silicon crystal silicon substrate. Anal Bioanal Chem 398:751–758
Gopinath SCB, Awazu K, Fujimaki M (2012) Waveguide-mode sensors as aptasensors. Sensors 12:2136–2151
Gopinath SCB, Hayashi K, Lee J, Kamori T, Dong C, Hayashi T, Kumar PKR (2013) Analyses of compounds that interfere the herpes simplex virus host receptor interactions using surface plasmon resonance. Anal Chem 85:10455–10462
Gopinath SCB, Lakshmipriya T, Awazu K (2014) Colorimetric detection of controlled assembly and disassembly of aptamers on unmodified gold nanoparticles. Biosens Bioelectron 51:115–123
Huang W, Bulusu S, Pal R, Zeng XC, Wang LS (2009) Structural transition of gold nanoclusters: from the golden cage to the golden pyramid. ACS Nano 3:1225–1230
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B 110:15700–15707
Lakshmipriya T, Fujimaki M, Gopinath SCB, Awazu K (2013a) Generation of anti-influenza aptamers using the systematic evolution of ligands by exponential enrichment for sensing applications. Langmuir 29:15107–15115
Lakshmipriya T, Fujimaki M, Gopinath SCB, Awazu K, Horiguchi Y, Nagasaki Y (2013b) A high-performance waveguide-mode biosensor for detection of factor IX using PEG-based blocking agents to suppress non-specific binding and improve sensitivity. Analyst 138:2863–2870
Lakshmipriya T, Fujimaki M, Gopinath SCB, Awazu K, Horiguchi Y, Nagasaki Y (2013c) A high-performance waveguide-mode biosensor for detection of factor IX using PEG-based blocking agents to suppress non-specific binding and improve sensitivity. Analyst 138:2863
Lakshmipriya T, Horiguchi Y, Nagasaki Y (2014) Co-immobilized poly(ethylene glycol)-block-polyamines promote sensitivity and restrict biofouling on gold sensor surface for detecting factor IX in human plasma. Analyst 139:3977–3985
Lakshmipriya T, Gopinath SCB, Tang T-H (2016) Biotin-streptavidin competition mediates sensitive detection of biomolecules in enzyme-linked immunosorbent assay. PLoS One 11:e0151153
Lee J, Ulmann PA, Han MS, Mirkin CA (2008) A DNA-gold nanoparticle-based colorimetric competition assay for the detection of cysteine. Nano Lett 8:529–533
Lei KF, Law WC, Suen YK, Li WJ, Ho HP, Lin C, Kong SK (2005) Bio-molecular and cellular detection using SPR sensor and all-transparent microfluidic platform. In: 2005 5th IEEE Conf Nanotechnol, vol. 1, pp 515–518
Li X, Nie Z, Cheng C (2010) Paper-based electrochemical ELISA. In: 14th International conference on miniaturized systems for chemistry and life sciences, Groningen, The Netherlands. pp 1487–1489
Liu J, Mazumdar D, Lu Y (2006) A simple and sensitive “dipstick” test in serum based on lateral flow separation of aptamer-linked nanostructures. Angew Chemie Int Ed 45:7955–7959
Liu Y, Yu J, Chen W, Liu D, Wang Z, Jiang X (2012) Cu2+ detection with gold nanoparticles by patterning colorimetric strips on a filter membrane assembled in a microfluidic chip. Chin J Chem 30:2047–2051
Liu B, Wu T, Yang X, Wang Z, Du Y (2014) Portable microfluidic chip based surface-enhanced raman spectroscopy sensor for crystal violet. Anal Lett 47:2682–2690
Liu G, Yang X, Li Y, Yang Z, Hong W, Liu J (2015) Continuous flow controlled synthesis of gold nanoparticles using pulsed mixing microfluidic system. Adv Mater Sci Eng 2015:160819
Luo C, Fu Q, Li H, Xu L, Sun M, Ouyang Q, Chen Y, Ji H (2005) PDMS microfluidic device for optical detection of protein immunoassay using gold nanoparticles. Lab Chip 5:726–729
Nagasaki Y (2011) Construction of a densely poly(ethylene glycol)-chain-tethered surface and its performance. Polym J 43:949–958
Nehl CL, Liao H, Hafner JH (2006) Optical properties of star-shaped gold nanoparticles. Nano Lett 6:683–688
Nomura K, Gopinath SCB, Lakshmipriya T, Fukuda N, Wang X, Fujimaki M (2013) An angular fluidic channel for prism-free surface-plasmon-assisted fluorescence capturing. Nat Commun 4:2855
Pallaoro A, Hoonejani MR, Braun GB, Meinhart CD, Moskovits M (2015) Rapid identification by surface-enhanced raman spectroscopy of cancer cells at low concentrations flowing in a microfluidic channel. ACS Nano 9:4328–4336
Park TJ, Kim HY, Lee J, Kim BI, Park JH, Kim KM, Yang EK, Ahn CW, Lee SY, Lee SJ (2009) Imprinted microfluidic device for biotin- spired detection of avian influenza virus. In: Thirteenth international conference on miniaturized systems for chemistry and life sciences, November, Jeju, Korea
Picciolini S, Mehn D, Morasso C, Vanna R, Bedoni M, Pellacani P, Marchesini G, Valsesia A, Prosperi D, Tresoldi C, Ciceri F, Gramatica F (2014) Polymer nanopillar–gold arrays as surface-enhanced raman spectroscopy substrate for the simultaneous detection of multiple genes. ACS Nano 8:10496–10506
Piorek BD, Lee SJ, Moskovits M, Meinhart CD (2012) Free-surface microfluidics/surface-enhanced Raman spectroscopy for real-time trace vapor detection of explosives. Anal Chem 84:9700–9705
Qu S, Li H, Peng T, Gao Y, Qiu J, Zhu C (2004) Optical nonlinearities from transverse plasmon resonance in gold nano-rods. Mater Lett 58:1427–1430
Rohrman BA, Leautaud V, Molyneux E, Richards-Kortum RR (2012) A lateral flow assay for quantitative detection of amplified HIV-1 RNA. PLoS One 7:e45611
SadAbadi H, Badilescu S, Packirisamy M, Wüthrich R (2013) Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens Bioelectron 44:77–84
Shiao Y-S, Chiu H-H, Wu P-H, Huang Y-F (2014) Aptamer-functionalized gold nanoparticles as photoresponsive nanoplatform for co-drug delivery. ACS Appl Mater Interfaces 6:21832–21841
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Yang J, Palla M, Bosco FG, Schmidt MS, Rindzevicius T, Boisen A, Ju J, Lin Q (2013) A microfluidic surface enhanced Raman spectroscopic biosensor using aptamer-functionalized nanopillars. In: 2013 Transducers Eurosensors XXVII 17th Int. conf. solid-state sensors, actuators microsystems, Trans Eurosens, vol. 2013, pp 1799–1802
Yuan Y, Gopinath SCB, Kumar PKR (2011) Regeneration of commercial biacore chips to analyze biomolecular interactions. Opt Eng 50:034402-1–034402-6
Zhang F, Li S, Cao K, Wang P, Su Y, Zhu X, Wan Y (2015) A microfluidic love-wave biosensing device for PSA detection based on an aptamer beacon probe. Sensors 15:13839–13850
Zordan MD, Grafton MMG, Acharya G, Reece LM, Aronson AI, Park K, Leary JF (2009) A microfluidic-based hybrid SPR/molecular imaging biosensor for the multiplexed detection of foodborne pathogens. Proc. SPIE 7167:716706–716710
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lakshmipriya, T., Hashim, U., Gopinath, S.C.B. et al. Microfluidic-based biosensor: signal enhancement by gold nanoparticle. Microsyst Technol 22, 2389–2395 (2016). https://doi.org/10.1007/s00542-016-3074-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-016-3074-1