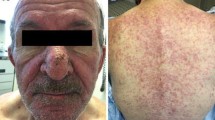
Abstract
Purpose
Inhibition of the vascular endothelial growth factor receptor (VEGFR) with tyrosine kinase inhibitors (TKIs) is associated with cutaneous adverse effects that increase patient morbidity. Our objective was to examine the skin toxicity profile of anti-VEGFR TKIs and determine the changing incidence in clinical trials.
Methods
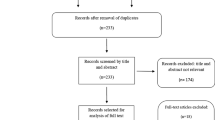
PubMed was queried for phase II or III trials of anti-VEGFR TKIs between 2000 and 2013 involving ≥50 patients. Adverse events were abstracted, with results presented in both fixed and random effects models. Odds ratios (OR) and 95 % confidence intervals (CIs) were estimated for studies with at least two arms.
Results
Across 82 included studies, all grades rash (OR, 2.68; 95 % CI, 2.45–2.94), hand-foot skin reaction (HFSR) (OR, 2.70; 95 % CI, 2.43–3.00), and pruritus (OR, 1.25; 95 % CI, 1.12–1.39) were associated with anti-VEGFR TKIs. Vandetanib had the highest incidence of rash (41 %), while sorafenib was most commonly associated with HFSR (37 %) and pruritus (14 %). The incidence of HFSR from 2000 to 2013 showed an upward trend (r 2 = 0.042, p = 0.10) and in sunitinib therapy increased significantly (r 2 = 0.237, p = 0.04).
Conclusion
The incidence of HFSR, rash, and pruritus varies considerably by drug. Our data suggest a continued need to address skin toxicities and improve reporting strategies.






Similar content being viewed by others
References
Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22:1276–1312
Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH (2009) Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res 15:1411–1416
Balagula Y, Rosen ST, Lacouture ME (2011) The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol 65:624–635
Breccia M, Carmosino I, Russo E, Morano SG, Latagliata R, Alimena G (2005) Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol 74:121–123
Chow LQ, Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25:884–896
Chu D, Lacouture ME, Fillos T, Wu S (2008) Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol 47:176–186
Chu D, Lacouture ME, Weiner E, Wu S (2009) Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer 7:11–19
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, Group TS (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM (2009) Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27:3312–3318
Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM (2009) Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27:1280–1289
Fischer A, Wu S, Ho AL, Lacouture ME (2013) The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs 31:787–797
Gomez P, Lacouture ME (2011) Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist 16:1508–1519
Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–3457
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Janusch M, Fischer M, Marsch WC, Holzhausen HJ, Kegel T, Helmbold P (2006) The hand-foot syndrome—a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol 16:494–499
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96:1788–1795
Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP (2008) A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26:127–132
Lacouture ME, Reilly LM, Gerami P, Guitart J (2008) Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 19:1955–1961
Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP (2008) Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 13:1001–1011
Lee JH, Chung YH, Kim JA, Shim JH, Lee D, Lee HC, Shin ES, Yoon JH, Kim BI, Bae SH, Koh KC, Park NH (2013) Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer 119:136–142
Lipworth AD, Robert C, Zhu AX (2009) Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 77:257–271
McTigue M, Murray BW, Chen JH, Deng YL, Solowiej J, Kania RS (2012) Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci U S A 109:18281–18289
Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F (2006) Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist 11:753–764
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, ** J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Nakano K, Komatsu K, Kubo T, Natsui S, Nukui A, Kurokawa S, Kobayashi M, Morita T (2013) Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn J Clin Oncol 43:1023–1029
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stähle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM, Investigators I (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496
Porta C, Paglino C, Imarisio I, Bonomi L (2007) Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med 7:127–134
Posadas EM, Limvorasak S, Sharma S, Figlin RA (2013) Targeting angiogenesis in renal cell carcinoma Expert Opin Pharmacother 14:2221–2236
Prasad V, Massey P, Fojo T (2014) Oral anticancer drugs: how limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol 32:1620–9
Ranieri G, Mammì M, Donato Di Paola E, Russo E, Gallelli L, Citraro R, Gadaleta CD, Marech I, Ammendola M, De Sarro G (2013) Pazopanib a tyrosine kinase inhibitor with strong anti-angiogenetic activity: a new treatment for metastatic soft tissue sarcoma Crit Rev Oncol Hematol 89:322–329
Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME (2012) Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab 97:1125–1133
Sahade M, Caparelli F, Hoff PM (2012) Cediranib: a VEGF receptor tyrosine kinase inhibitor. Future Oncol 8:775–781
Shin JW, Chung YH (2013) Molecular targeted therapy for hepatocellular carcinoma: current and future. World J Gastroenterol 19:6144–6155
Shukla S, Chen ZS, Ambudkar SV (2012) Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat 15:70–80
Smaglo BG, Hwang J (2013) Continuum of care with anti-angiogenic therapies in metastatic colorectal cancer. J Gastrointest Oncol 4:299–307
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 23 Nov 2014
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D (2011) Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129:245–255
Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, Kuo TT, Hsieh JJ, Chang JW (2008) Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 158:592–596
Acknowledgments
We would like to thank Drs. Mark Udey and Heidi Kong of the NCI for assistance in editing this manuscript and Dr. Tito Fojo for his general advice regarding this project.
Author attribution
All authors had full access to all of the data in the study and take responsibility for the integrity of the data. Study concept and design: Massey, Cowen. Acquisition of data: Massey, Okman. Analysis and interpretation of data: all authors. Drafting of the manuscript: Massey, Okman, Cowen. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Wilkerson. Obtained funding: N/A. Administrative, technical, or material support: N/A. Study supervision: Cowen.
Funding/support/role of the sponsors
This research was made possible in part by the Intramural Research Program of the NIH, National Cancer Institute.
Financial disclosure
None reported.
Conflict of interest
The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1994 kb)
Rights and permissions
About this article
Cite this article
Massey, P.R., Okman, J.S., Wilkerson, J. et al. Tyrosine kinase inhibitors directed against the vascular endothelial growth factor receptor (VEGFR) have distinct cutaneous toxicity profiles: a meta-analysis and review of the literature. Support Care Cancer 23, 1827–1835 (2015). https://doi.org/10.1007/s00520-014-2520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2520-9