Summary
Background
The use of venovenous extracorporeal membrane oxygenation (VV-ECMO) as a rescue therapy in severe acute respiratory distress syndrome (ARDS) has become well established; however, the affirmation of evidence on VV-ECMO application and the analysis of patient outcomes after VV-ECMO treatment for ARDS continues. The aim of the study is to identify variables that affected the outcome of patients treated with VV-ECMO for severe ARDS outside a major ECMO center.
Methods
The study included adult patients with severe ARDS treated with ECMO at a tertiary care hospital in Zagreb, Croatia between October 2009 and July 2014. Patients were recruited from a prospective database.
Results
The study enrolled 40 patients, 20 of whom had H1N1-induced ARDS. The hospital mortality was 38%. The difference in mortality and long-term outcome in H1N1-induced ARDS as compared to non-H1N1-induced ARDS was not significant. Variables associated with mortality included immunosuppression, shock at time of admission, acute renal failure, occurrence of heparin-induced thrombocytopenia antibodies, nosocomial sepsis and duration of ECMO.
Conclusions
The results of our study indicate that ECMO use in severe ARDS is feasible with low mortality and identify or assert the variables associated with adverse outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venovenous extracorporeal membrane oxygenation (VV-ECMO) has become established in the treatment of the most severe forms of acute respiratory distress syndrome (ARDS) during the last decades [1–3]; however, as only one randomized control trial (CESAR) has been conducted and published so far, the collection of evidence for ECMO use in ARDS continues [4].
The mortality of mechanically ventilated patients with severe ARDS with a pO2/FiO2 (partial pressure of oxygen in alveoles/oxygen inspiratory fraction set on the ventilator) ratio of <100 is approximately 60% [5]. Extracorporeal Life Support Organization (ELSO) guidelines predict a mortality of 80% if this ratio is <100 on a FiO2 of 90%. These mortality data should be used to compare the outcome of patients treated with ECMO for ARDS among different trials if the control group is not included.
Our respiratory ECMO center was established in October 2009 in the midst of the H1N1 pandemic with the intention to help patients with severe H1N1 ARDS. Training of our staff was provided by local cardiothoracic surgeons and perfusionists. After initial training we enhanced our practical and theoretical knowledge at renowned European ECMO centers (Lueven, Karolinska and Regensburg). For the first 2 years we had perfusionists on call. Afterwards we were completely independent with intensive care specialists and nurses responsible for all the aspects of ECMO management. Our ECMO program is available around the clock since the beginning and is responsible for respiratory ECMO support in Croatia. In 2013 we became the only certified respiratory ECMO center in Croatia with the decision of our national Ministry of Health.
The primary objective of this study was to report a single center experience with VV-ECMO treatment for severe ARDS outside a major ECMO center and to analyze the variables that affected the outcome. Furthermore, as a secondary objective local guidelines for the treatment of severe ARDS were established. We believe that our trial contributed further evidence to the existing pool of knowledge about the use of VV-ECMO in ARDS.
Patients, materials and methods
Patients
The study included 40 adult patients with ARDS treated with ECMO at the Zagreb University Hospital for Infectious Diseases, a tertiary care hospital in Croatia, in the period between October 2009 and June 2014. Patients with H1N1 ARDS received antiviral treatment with oseltamivir at a dose of 2 × 150 mg. Empirical antimicrobial treatment consisted of ceftriaxone (1 × 2 g intravenously daily) and azithromycin (1 × 500 mg intravenously daily). Steroids were administered at low dosage (2 × 100 mg hydrocortisone intravenously daily) in all patients; however, as steroid treatment in H1N1 ARDS could be harmful, this practice was terminated in patients that followed [6].
The non-H1N1 group was empirically treated with ceftriaxone (1 × 2 g intravenously) and azithromycin (1 × 500 mg intravenously) if the diagnosis was simple community acquired pneumonia. Immunosuppressed patients with bacterial pneumonia were treated with piperacillin-tazobactam (4 × 4.5 g intravenously) or cefepime (2 × 2 g intravenously). Septic patients were empirically treated with broad spectrum antimicrobial coverage with de-escalation after the antimicrobial susceptibility testing was performed. The remaining patients were treated according to the diagnosis (e.g. acute eosinophilic pneumonia with steroids, aspiration pneumonitis with steroids and antibiotics, Goodpasture syndrome with plasma exchange, steroids and cyclophosphamide and bleomycin-induced lung injury with steroids). All patients received a neuromuscular blockade for the first 48 h [7]. Prone positioning was not performed prior to or during ECMO and treatment with nitric oxide and prostaglandins was not employed due to lack of evidence of efficacy. Informed consent was obtained from relatives of all patients included in the study. Institutional ethical review board approved the investigation.
Data
Data were acquired from a prospectively collected database of patients treated with ECMO for acute respiratory failure. Variables included in the analysis were age, gender, mortality, etiology, immunosuppression (e.g. cirrhosis, hematological malignancy, immunosuppressive treatment and hypogammaglobulinemia), presence of diabetes mellitus, body mass index, acute physiology and chronic health evaluation II (APACHE II) score, Murray score, pO2/FiO2 ratio, oxygenation index (FiO2 × mean airway pressure/pO2), pCO2, shock at admission (systolic blood pressure SBP <90 mm Hg, mean arterial pressure <70 mm Hg or an SBP decrease >40 mm Hg in adults or less than two standard deviations below normal for age with concomitant use of vasopressor treatment – norepinephrine >0.1 µg/kg/min), duration of mechanical ventilation before ECMO, duration of ECMO, occurrence of acute renal failure (according to the RIFLE classification system [8]), hemolysis (free hemoglobin >50 mg/dl), possible heparin-induced thrombocytopenia HIT, defined by the detection of PF-4 antibodies by ELISA and low platelet counts, significant hemorrhage (≥1000 ml of red blood cell transfusion per complication) and nosocomial sepsis. For the long-term outcome assessment the Karnofsky performance scale score was used.
ECMO
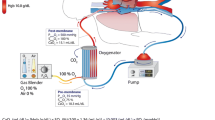
All ECMO circuits were venovenous with centrifugal pumps and were biocoated. Of the patients 27 had a femorojugular bypass with drainage at the femoral site. The remaining 13 patients had a femorofemoral bypass. Biomedicus cannulas, Biomedicus BP-80 pump, Medtronic I‑4500 and Eurosets (Medolla, Italy) ADULT ECMO oxygenators and Medtronic (Dublin, Ireland) BIOtherm heat exchanger were used from November 2009 to September 2013. After September 2013 in 14 patients we used the adult permanent life support (PLS) system by Maquet (Gettinge group, Rastatt, Germany) with all the components of the set included. According to the ELSO guidelines the indications for ECMO include a pO2/FiO2 ratio <100, Murray score of 3–4 and/or refractory hypercapnia with pH <7.2. Cannulations were percutaneous with the Seldinger technique in all patients. Cannula size was individually determined with the average drainage cannula size being 23 Fr. and 21 Fr. for male and female patients, respectively. Average return cannula size was 21 Fr. and 19 Fr. for males and females, respectively. If additional blood flow was necessary we augmented drainage with insertion of an additional cannula to the available femoral vein with a Y‑connector.
Heparin infusion was titrated according to the bedside activated clotting time (ACT) measurements that were performed every hour. The ACT values were targeted at the range between 170–180 s. Three patients were treated with fondaparinaux 2.5 mg daily due to positive HIT antibodies (PF-4) and two other patients died before the results for the HIT antibodies were disclosed. If indicated, a continuous renal replacement therapy (CRRT) circuit was integrated in the ECMO circuit between the centrifugal pump and the oxygenator. The ECMO blood and sweep gas flow was adjusted to achieve normocarbia, normal pH, oxygen saturation above 90% and normal lactate levels. Ventilation parameters during ECMO were based on pressure controlled ventilation with peak pressure of 25 cmH2O, PEEP from 8–10 cmH2O, FiO2 21–30% and inspiration to expiration ratio of 1:1(2). Spontaneous breathing was supported with additional pressure support of 10–15 cm H2O. The goal of ventilation parameters was to reduce ventilator-induced lung injury.
Patients were sedated with midazolam infusion and analgesia was achieved with fentanyl infusion. Weaning trial was tried when ventilation and oxygenation parameters showed improvement from the baseline. The patient was decannulated when tidal volumes approximated 500 ml on the pressure controlled ventilation with upper pressure of 20–25 cm H2O, when CO2 was normal without sweep gas on and when pO2 was 90–100 mm Hg with the FiO2 of 70% on the ventilator and without ECMO.
Statistics
Continuous variables are presented as the median, the 25th and the 75th percentiles. Categorical variables are presented as frequencies and percentages. Univariate analysis tested the statistical significance of the difference in outcome variables between the survivors and non-survivors with Fisher’s two-tailed exact test for categorical and the Mann-Whitney test for continuous variables. Both tests were used due to non-parametrical distribution of the data. Survivors were patients who were alive 3 months after discharge from hospital. P-values of less than 0.05 were considered to indicate a statistical significance in all statistical tests. For statistical analysis SAS software for Windows, version 9.3. (SAS Institute) was used.
Results
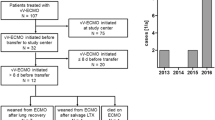
The study included 40 consecutive adult patients with ARDS treated with VV-ECMO at a single center. The patient cohort had a median age of 48.5 years and a mortality rate of 38% (15 out of 40). The median time on ECMO was 192 h, somewhat shorter than usual, probably due to early mortality in 3 patients that affected the median ECMO time of our relatively small number of patients. Further detailed demographics, clinical characteristics and univariate analysis according to the survival of included patients are presented in Table 1.
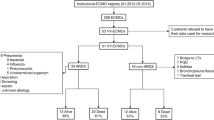
A total of 20 patients had H1N1 infections confirmed by real-time polymerase chain reaction (RT-PCR) testing in bronchoalveolar lavage or tracheal aspirate specimens. In the non-H1N1 group 12 patients had bacterial pneumonia (4 Streptococcus pneumoniae, 3 of unknown etiology, 2 Legionella pneumophila, 1 Pseudomonas aeruginosa, 1 Klebsiella pneumoniae, and 1 Acinetobacter baumannii) and 3 patients had sepsis (1 Staphylococcus aureus and 2 Escherichia coli). Of the remaining five patients one each had adenoviral pneumonia, acute eosinophilic pneumonia, aspiration pneumonitis, Goodpasture syndrome and bleomycin-induced lung injury.
The severity of disease and ARDS are demonstrated by the high APACHE II score and oxygenation index as well as the low median pO2/FiO2 ratio. According to the median body mass index (BMI) of 27.1, the patients were overweight but not obese. Of the patients nine (23%) were immunocompromised: two had hematological malignancy, two had hypogammaglobulinemia, two had cirrhosis and three received immunosuppression treatment. Of the patients nine (23%) had significant bleeding episodes that required 1000 ml of red blood cell transfusions or more until the bleeding was controlled. Hemorrhage symptoms were present at cannula insertion site in two patients, three had hematemesis and in four patients the hemorrhage was due to hematuria, hematochezia, intra-abdominal and intrathoracic hemorrhage, respectively. Other patients required red blood cell transfusions occasionally to achieve hematocrit levels above 30%. Of the patients 16 (40%) had significant hemolysis that required investigation: in severe cases (free hemoglobin more than 500 mg/dl) it required either centrifugal pump head change or the change of the complete ECMO circuit from cannula to cannula for suspected thrombus formation within the circuit. The majority of patients were placed on CRRT for fluid balance optimization and 16 also had acute renal failure. Anti-HIT antibodies were detected by ELISA in three patients (8%) and nosocomial bloodstream infections (BSI) developed in 17 patients (7 Staphylococcus epidermidis, 7 Acinetobacter baumanii, 2 Pseudomonas aeruginosa nd 1 Candida albicans).
In order to detect possible variables associated with mortality we performed univariate analysis that is presented in Table 1. Baseline severity of the ARDS was not significantly different between survivors and non-survivors as revealed by the oxygenation index, pO2/FiO2 ratio, pCO2 and Murray score. The APACHE II score was higher in the non-survivor group; however, the difference only indicated a tendency and was not significant (p = 0.08). Immunosuppression, acute renal failure and shock at admission were more frequent in non-survivors as was the occurrence of HIT, nosocomial sepsis and hemolysis. Duration of mechanical ventilation prior to ECMO initiation was comparable between groups as was BMI. The duration of VV-ECMO support was significantly longer in non-survivors, this is important because the incidence of complications increased with the time on ECMO. We also analyzed the influence of H1N1 influenza etiology of ARDS on the outcome and the mortality between the groups was comparable, 7/20 vs. 8/20 for the H1N1 and the non H1N1 groups, respectively.
Of non-survivors four patients died after discontinuation of ECMO, one from septic shock, one from pulmonary embolism and two patients died due to intracerebral hemorrhage. Of the remaining 11 patients who died while on ECMO, 9 presumably died of septic shock (sepsis was confirmed in 7 patients), 1 patient died of myocardial infarction and 1 of suspected pulmonary embolism. Long-term outcome of the survivors was evaluated with the Karnofsky performance scale 3 months after discharge from hospital. Results revealed a median Karnofsky performance scale score of 90% and were both satisfactory and expected as the patient cohort was relatively young.
Discussion
The results of our study revealed that when indicated, VV-ECMO can be a life-saving procedure for the most severe form of ARDS. Our patients, although severely ill on admission with a median pO2/FiO2 ratio of 61.3, had a mortality rate of 38% (15/40) indicating that satisfactory survival is feasible with VV-ECMO treatment despite the procedural complexity. Our trial also contributed to the development of the local guidelines for the treatment of severe ARDS as we were able to provide information on when to transfer a patient to the respiratory ECMO center in order to avoid transport on ECMO. Today indications for the transfer of patients to our ECMO center are: pO2/FiO2 <100 and oxygenation index >20 but not all patients receive ECMO treatment as some improve on conventional ventilation. If the patient already has an indication for ECMO or cannot be safely transported on mechanical ventilation, our mobile team retrieves the patient from the referring hospital.
The long-term outcome of our patients was encouraging with a median Karnofsky performance scale score of 90% indicating minor signs of disease; however, all patients received further medical rehabilitation after hospital discharge. Our study also asserted the evidence that hemolysis is a significant variable for adverse outcome in ECMO patients and requires immediate investigation when detected [9]. A higher incidence of hemolysis in our cohort of patients was probably due to older ECMO circuit we used until 2013 (Medtronic oxygenator and Biomedicus centrifugal pumps) that is more prone to thrombus formation and hemolysis. Consequently, circuits had to be changed more frequently. Furthermore, we used Biomedicus cannulas without side perforations for suction and had more negative pressure cannula shaking that increases hemolysis. An immunosuppressive state as defined in the study, significantly correlated with the mortality as was detected in previous studies [10]; however, the immunosuppressed group was heterogeneous and small in size, thus preventing any inference to the adverse outcome. Nosocomial sepsis confirmed by positive blood cultures and signs of systemic inflammatory response syndrome (SIRS) as well as detection of HIT–PF 4 antibodies were significantly more common in non-survivors; however, this could be related to longer ECMO duration in the same group as most patients died while on ECMO. In the survivors group, most patients were weaned from ECMO by day 10, thus it seems prudent to escalate the search for new infectious and non-infectious complications that can deteriorate oxygenation after this period. Acute renal failure significantly influenced the outcome as was determined by previous investigations [11]. The presence of shock at admission was determined in 23% of patients (9/40) and was indicative of the adverse outcome with significant impact on mortality; however, in eight patients the shock resolved after ECMO initiation and the decrease in intrathoracic pressure, while one patient died in refractory septic shock (indicating that the true shock was present only in one patient). This fact confirms that VV-ECMO is the first-choice therapy in ARDS patients with preserved myocardial function even if shock with vasoactive drug treatment is present on admission. Unlike in the PRESERVE study, in our patients the BMI did not affect mortality, probably because our sample size was too small to detect the difference [10].
The severity of lung failure prior to ECMO treatment had no influence on the mortality as has been previously described [7]. This indicates that the outcome of ARDS patients treated with VV-ECMO depends more on the ability to etiologically treat the condition that induced ARDS and on the ability of the lungs to heal, than on the severity of the initial insult to the lungs. Further advantages of ECMO that probably affect mortality in severe ARDS are lung rest that enhances the healing process by avoiding ventilator-induced lung injury and capability of ECMO to completely substitute lung function.
The limitations of this study are substantial and mostly due to the small number of patients that prevented more robust analysis and inference of the results. Furthermore, without a control group it is obvious that our trial intention was not to advocate VV-ECMO treatment over conventional ventilation in severe ARDS; however, it is obvious that the use of VV-ECMO in ARDS will continue in the future and that the body of evidence must be expanded, especially as the incidence of ARDS requiring ECMO is low. Furthermore, it is difficult to organize a randomized control trial in patients with severe ARDS as it is unacceptable to randomize patients to the control group who really need ECMO. In these circumstances, despite the obvious limitations the information obtained by our study indicates that ECMO seems to be feasible and despite a considerable rate of complications, can have satisfactory outcome in terms of mortality. High expectations of the ongoing randomized controlled trial EOLIA are present in the whole ECMO community. It is an international multicenter, randomized, open trial that will evaluate the impact of ECMO on the morbidity and mortality associated with ARDS and we believe that it would make selection of patients with ARDS that would benefit from ECMO treatment more accurate.
According to the available evidence, the results obtained from this study and a 5-year experience in a referral center for respiratory ECMO in Croatia, our conclusions are in accordance with the recently published literature [12, 13]. According to those conclusions and in order to maximize its positive effects in ARDS, ECMO should be reserved for patients with persistent pO2/FiO2 ratio of around 60 and with CO2 retention resulting in significant respiratory acidosis (pH <7.15). Furthermore, relentless and prompt search for the ARDS etiology is mandatory in order to treat, if possible, the condition that induced ARDS, as the time we are able to safely provide ECMO support is usually limited. In patients presenting with variables that have significant impact on adverse outcome at admission, such as acute renal failure, shock or immunosuppression, an even more aggressive quest for the etiology of ARDS should be performed. For example, while the patient was on ECMO we performed renal biopsy, fortunately without any complications, to confirm the diagnosis of Goodpasture syndrome within 12 h and to justifiably administer cyclophosphamide treatment that is fraught with complications. Consequently, while the patient is on ECMO our first objective should be to take every measure necessary that will provide the best possible environment for the lungs to heal.
With such thorough and individual approaches towards patients with severe ARDS we will be able to utilize ECMO in the most purposeful manner, with avoidance of unnecessary ECMO support with all the possible complications and the best cost-benefit ratio. Furthermore, with such prudent application of VV-ECMO, we have an additional tool to achieve the main objective, the lowest possible mortality of ARDS.
References
Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888–95.
Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–68.
Roch A, Hraiech S, Masson E, et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med. 2014;40(1):74–83.
Peek GJ, Mugford M, Tiruvoipati R, et al. CESAR: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–63.
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–16.
Brun-Buisson C, Richard JC, Mercat A, Thiébaut AC, Brochard L. REVA-SRLF A/H1N1v 2009 Registry Group. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;183(9):1200–6.
Villar J, Pérez-Méndez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting – a prospective, multicenter validation study. Intensive Care Med. 2013;39(4):583–92.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, the ADQI workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group.Crit Care. 2004;8(4):R204–R212.
Gbadegesin R, Zhao S, Charpie J, Brophy PD, Smoyer WE, Lin JJ. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24(3):589–95.
Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704–13.
Hsiao CC, Chang CH, Fan PC, et al. Prognosis of patients with acute respiratory distress syndrome on extracorporeal membrane oxygenation: the impact of urine output on mortality. Ann Thorac Surg. 2014;97(6):1939–44.
Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–96.
Richard C, Argaud L, Blet A, Boulain T, Contentin L, Dechartres A. Extracorporeal life support for patients with acute respiratory distress syndrome: report of a Consensus Conference. Ann Intensive Care. 2014;24(4):15. doi:10.1186/2110-5820-4-15.
Acknowledgements
We are thankful to the staff at the Department of Intensive Care Medicine and Neuroinfections at the University Hospital for Infectious Diseases “Dr. Fran Mihaljević” for their dedication and expertise. We especially thank the following persons who were personally responsible for ECMO treatment: Mirjana Vranjican, Ines Filko, Anica Jasenko, Tamara Glumpak, Vesna Horvat, Andrea Deduš, Dragan Lepur, Marija Santini, Vladimir Kra**ović and Dinko Raffanelli.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Kutleša, A. Novokmet, R. Josipović Mraović, and B. Baršić declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kutleša, M., Novokmet, A., Josipović Mraović, R. et al. Venovenous extracorporeal membrane oxygenation for ARDS: outcome analysis of a Croatian referral center for respiratory ECMO. Wien Klin Wochenschr 129, 497–502 (2017). https://doi.org/10.1007/s00508-016-1109-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-016-1109-3