Summary
Background
Contamination of surfaces by spores of Clostridium difficile is a major factor influencing the spread of healthcare-associated C. difficile infection. The aim of this study was to test the effect of an automated room disinfection system that provides an aerosol of 7.5 % hydrogen peroxide (H2O2) disinfectant, on spores of two different strains of C. difficile, and to evaluate the impact of biological soiling on the efficacy of H2O2 disinfection.
Material and method
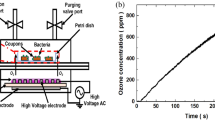
The strains used were a C. difficile PCR ribotype 027 and a C. difficile ATCC 9689. Spore suspensions of each strain were applied to ceramic tiles and exposed to aerosolized H2O2 at different locations in a test room. Biological soiling was simulated by bovine serum albumin and sheep erythrocytes. At set time points spores were recovered, plated onto Columbia 5 % sheep blood agar, and surviving bacteria were counted as colony-forming units (cfu).
Results
No viable spores of either strain were recovered after a 3 h exposure to gaseous H2O2. Spores located inside a drawer showed recovery of approximately 1E5 cfu/ml for C. difficile ribotype 027 after 1 h. In the presence of organic matter, a more than fivefold log reduction compared with not exposed controls could be observed for spores of either strain tested.
Conclusion
Appropriate decontamination of surfaces exposed to spores of C. difficile is challenging for conventional cleaning methods. Aerosolized H2O2 delivered by automated room disinfection systems could possibly improve surface decontamination and thereby reduce transmission of healthcare-associated C. difficile infection. Also in the presence of organic matter H2O2 disinfection appears to be an effective adjunct for decontamination of environmental surfaces.
Zusammenfassung
Hintergrund
Kontamination von Oberflächen mit Sporen von Clostridium difficile ist ein wesentlicher Faktor für die Entstehung nosokomialer C. difficile Infektionen. Zum Zwecke der Raumdekontamination stehen automatisierte Systeme zur Verfügung, welche Lösungen von Wasserstoffperoxid vernebeln. Das Desinfektionspotential eines solchen Systems wurde hinsichtlich seiner Wirkung auf unterschiedliche C. difficile Stämme, in An- und Abwesenheit organischer Verunreinigung, untersucht.
Material und Methode
Es wurden Sporensuspensionen zweier C. difficile Stämme, Ribotyp 027 sowie ATCC 9689, auf Keramikkacheln aufgebracht und an verschiedenen Positionen eines Versuchsraumes platziert. Die anschließende Begasung erfolgte mit einem Aerosol einer 7,5 %igen H2O2 Lösung. Zur Simulation organischer Verunreinigung wurden den Sporensuspensionen Rinderalbumin sowie Schaferythrozyten beigemengt. Zu definierten Zeitpunkten erfolgten eine Resuspendierung der Sporen und die Anzüchtung rekultivierbarer Bakterien.
Ergebnisse
Nach dreistündiger Begasung konnten keine lebensfähigen Sporen beider C. difficile Stämme resuspendiert werden. Der sporizide Effekt war geringer auf Sporen, die im Inneren einer Lade platziert waren; hier ließen sich nach einer Stunde Begasung noch 1E5 KBE/ml C. difficile RT 027 rekultivieren. Bei Simulation organischer Verunreinigung konnte eine über fünffache log-Reduktion im Vergleich zu nicht exponierten Kontrollen beobachtet werden.
Schlussfolgerung
Die Vernebelung eines Aerosols von Wasserstoffperoxid hat das Potential, die Oberflächenkontamination mit C. diffile zu reduzieren und somit die Übertragung nosokomialer C. difficile Infektionen hintanzuhalten. Auch bei organischer Verunreinigung scheint die Begasung mit H2O2 eine wirksame Ergänzung zur herkömmlichen Oberflächendekontamination zu sein.


Similar content being viewed by others
References
Schmid D, Kuo HW, Simons E, Wenisch J, Allerberger F, Wenisch C. All-cause mortality in hospitalized patients with infectious diarrhea: Clostridium difficile versus other enteric pathogens in Austria from 2008 to 2010. J Infect Public Health. 2014;7(2):133–44.
McGowan AP, Lalayiannis LC, Sarma JB, et al. Thirty-day mortality of Clostridium difficile infection in a UK National Health Service Trust between 2002 and 2008. J Hosp Infect. 2011;77(1):11–5.
Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl. 2):1–26.
Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect. 2012;18(Suppl. 6):5–12.
Rutala WA, Weber DJ. Are room decontamination units needed to prevent transmission of environmental pathogens? Inf Control Hosp Epidemiol. 2011;32(8):743–7.
Kuijper EJ, Coignart B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl. 6):2–18.
Merrigan M, Venugopal A, Mallozzi M, et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol. 2010;192(19):4904–11.
Indra A, Huhulescu S, Fiedler A, et al. Outbreak of Clostridium difficile 027 infection in Vienna, Austria 2008–2009. Euro Surveill. 2009;14(17):pii=19186. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19186. Accessed 05 Mar 2014.
Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32(7):687–99.
Otter JA, French GL. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J Clin Microbiol. 2009;27(1):205–7.
Donskey CJ. Preventing transmission of Clostridium difficile: is the answer blowing in the wind? Clin Infect Dis. 2010;50(11):1458–61.
Maillard JY. Innate resistance to sporicides and potential failure to decontaminate. J Hosp Infect. 2011;77(3):204–9.
Otter JA, Yezli S, Perl TM, et al. The role of ‘no-touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect. 2013;83(1):1–13.
Andersen BM, Rasch M, Hochlin K. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect. 2006;62(2):149–55.
Finnegan M, Denyer SP, McDonnell G, et al. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother. 2010;65(10):2108–15.
Bereket W, Hemalatha K, Getenet B, et al. Update on bacterial nosocomial infections. Eur Rev Med Pharmacol Sci. 2012;16(8):1039–44.
Stiefel U, Cadnum JL, Eckstein BC, et al. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect Control Hosp Epidemiol. 2011;32(2):185–7.
Falagas ME, Thomaidis PC, Kotsantis IK, et al. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect. 2011;78(3):171–7.
Walder M, Holmdahl T. Reply to Roberts. Infect Control Hosp Epidemiol. 2012;33(3):312–3.
Shapey S, Machin K, Levi K, et al. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J Hosp Infect. 2008;70(2):136–41.
Barbut F, Menuet D, Verachten M, et al. Comparison of the efficacy of a hydrogen peroxide dry mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect Control Hosp Epidemiol. 2009;30(6):507–14.
Havill NL, Moore BA, Boyce JM. Comparison of the microbiological efficacy of hydrogen peroxide vapor and ultraviolet light processes for room decontamination. Infect Control Hosp Epidemiol. 2012;33(5):507–12.
Barbut F, Yezli S, Otter JA. Activity in vitro of hydrogen peroxide vapour against Clostridium difficile spores. J Hosp Infect. 2012;80(1):85–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steindl, G., Fiedler, A., Huhulescu, S. et al. Effect of airborne hydrogen peroxide on spores of Clostridium difficile . Wien Klin Wochenschr 127, 421–426 (2015). https://doi.org/10.1007/s00508-014-0682-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-014-0682-6