Abstract
Key message
Long-term exposure of sweetgum trees to elevated atmospheric CO 2 concentrations significantly shifted inner bark (phloem) and outer bark (rhytidome) chemical compositions, having implications for both defense and nutrient cycling.
Abstract
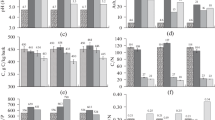
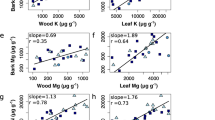
Changes in plant tissue chemistry due to increasing atmospheric carbon dioxide (CO2) concentrations have direct implications for tissue resistance to abiotic and biotic stress while living, and soil nutrient cycling when senesced as litter. Although the effects of elevated CO2 concentrations on tree foliar chemistry are well documented, the effects on tree bark chemistry are largely unknown. The objective of this study was to determine the effects of a long-term elevated CO2 treatment on the contents of individual elements, extractives, ash, lignin, and polysaccharide sugars of sweetgum (Liquidambar styraciflua L.) bark. Trees were harvested from sweetgum plots equipped with the Free-Air CO2 Enrichment (FACE) apparatus, receiving either elevated or ambient CO2 treatments over a 12-year period. Whole bark sections were partitioned into inner bark (phloem) and outer bark (rhytidome) samples before analysis. Principal component analysis, coupled with either Fourier transform infrared spectroscopy or pyrolysis–gas chromatography–mass spectrometry data, was also used to screen for differences. Elevated CO2 reduced the N content (0.42 vs. 0.35 %) and increased the C:N ratio (109 vs. 136 %) of the outer bark. For the inner bark, elevated CO2 increased the Mn content (470 vs. 815 mg kg−1), total extractives (13.0 vs. 15.6 %), and residual ash content (8.1 vs. 10.8 %) as compared to ambient CO2; differences were also observed for some hemicellulosic sugars, but not lignin. Shifts in bark chemistry can affect the success of herbivores and pathogens in living trees, and as litter, bark can affect the biogeochemical cycling of nutrients within the forest floor. Results demonstrate that increasing atmospheric CO2 concentrations have the potential to impact the chemistry of temperate, deciduous tree bark such as sweetgum.




Similar content being viewed by others
References
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment FACE? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Anttonen S, Manninen A-M, Saranpää P, Kainulainen P, Linder S, Vapaavuori E (2002) Effects of long-term nutrient optimization on stem wood chemistry in Picea abies. Trees 16:386–394
Boltersdorf SH, Pesch R, Werner W (2014) Comparative use of lichens, mosses and tree bark to evaluate nitrogen deposition in Germany. Environ Pollut 189:43–53
Calfapietra C, Angelis PD, Gielen B, Lukac M, Moscatelli MC, Avino G, Lagomarsino A, Polle A, Ceulemans R, Scarascia Mugnozza G, Hoosbeek M, Cotrufo MF (2007) Increased nitrogen-use efficiency of a short-rotation poplar plantation in elevated CO2 concentration. Tree Physiol 27:1153–1163
Chen H, Ferrari C, Angiuli M, Yao J, Raspi C, Bramanti E (2010) Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohydr Polym 82:772–778
Choong ET, Fogg PJ (1976) Extractives content and cell-wall specific gravity in sweetgum. LSU Wood Utilization Note #30, p 4
Davis MW (1998) A rapid modified method for compositional carbohydrate analysis of lignocellulosics by high pH anion-exchange chromatography with pulsed amperometric detection (HPAEC/PAD). J Wood Technol 18(2):235–252
Duca D, Riva G, Pedretti EF, Toscano G (2014) Wood pellet quality with respect to EN 14961-2 standard and certifications. Fuel 135:9–14
Eberhardt TL (2012) Impact of industrial source on the chemical composition of loblolly pine bark. For Prod J 62:516–519
Eberhardt TL (2013) Longleaf pine inner bark and outer bark thicknesses: measurement and relevance. South J Appl For 37:177–180
Eberhardt TL, Catallo WJ, Shupe TF (2010) Hydrothermal transformation of Chinese privet seed biomass to gas-phase and semi-volatile products. Bioresour Technol 101:4198–4204
Effland MJ (1977) Modified procedure to determine the acid-insoluble lignin in wood and pulp. Tappi J 60(10):143–144
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–376
Gall R, Landolt W, Schleppi P, Michellod V, Bucher JB (2002) Water content and bark thickness of Norway spruce (Picea abies) stems: phloem water capacitance and sap flow. Tree Physiol 22:613–623
Garten CT, Iversen CM, Norby RJ (2011) Litterfall 15N abundance indicates declining soil nitrogen availability in a free air CO2-enrichment experiment. Ecology 92:133–139
Gielen B, Ceulemans R (2001) The likely impact of rising atmospheric CO2 on natural and managed Populus: a literature review. Environ Pollut 115:335–358
Gilman EF, Watson DG (1993) Liquidambar styraciflua—sweetgum. Fact Sheet ST-358, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, p 4
Hatfield R, Fukushima RS (2005) Can lignin be accurately measured? Crop Sci 45:832–839
Hillis WE (1962) Wood extractives and their significance to the pulp and paper industries. Academic Press, New York
IPCC (2007) Summary for policy makers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, UK, pp 1–18
Iversen CM (2010) Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357
Johnson DW, Cheng W, Joslin JD, Norby RJ, Edwards NT, Todd DE Jr (2004) Effects of elevated CO2 on nutrient cycling in a sweetgum plantation. Biogeochemistry 69:379–403
Kaakinen S, Kostiainen K, Ek F, Saranpää P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E (2004) Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Glob Chang Biol 10:1513–1525
Kilpeläinen A, Gerendiain AZ, Luostarinen K, Peltola H, Kellomäki S (2007) Elevated temperature and CO2 concentration effects on xylem anatomy of Scots pine. Tree Physiol 27:1329–1338
Kim K, Labbé N, Warren JM, Elder T, Rials TG (2015) Chemical and anatomical changes in Liquidambar styraciflua L. xylem after long term exposure to elevated CO2. Environ Pollut 198:179–185
Körner C (2006) Plant CO2 responses: an issue of definition, time and resource supply. New Phytol 172:393–411
Kostiainen K, Kaakinen S, Warsta E, Kubiske ME, Nelson ND, Sober J, Karnosky DF, Saranpää P, Vapaavuori E (2008) Wood properties of trembling aspen and paper birch after 5 years of exposure to elevated concentrations of CO2 and O3. Tree Physiol 28:805–813
Kostiainen K, Kaakinen S, Saranpää P, Sigurdsson BD, Lundqvist S-O, Linder S, Vapaavuori E (2009) Stem wood properties of mature Norway spruce after 3 years of continuous exposure to elevated [CO2] and temperature. Glob Chang Biol 15:368–379
Labosky P Jr (1979) Chemical constituents of four southern pine barks. Wood Sci 12(2):80–85
Lestander TA, Lundström A, Finell M (2012) Assessment of biomass functions for calculating bark proportions and ash contents of refined biomass fuels derived from major boreal tree species. Can J For Res 42:59–66
Lindsey K, Johnson A, Kim P, Jackson S, Labbé N (2013) Monitoring switchgrass composition to optimize harvesting periods for bioenergy and value-added products. Biomass Bioenergy 56:29–37
Loladze I (2002) Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry. Trends Ecol Evol 17(10):457–461
López-González D, Fernandez-Lopez M, Valverde JL, Sanchez-Silva L (2014) Gasification of lignocellulosic biomass char obtained from pyrolysis: kinetic and evolved gas analyses. Energy 71:456–467
Luo Z-B, Calfapietra C, Liberloo M, Scarascia-Mugnozza G, Polle A (2006) Carbon partitioning to mobile and structural fractions in poplar wood under elevated CO2 (EUROFACE) and N fertilization. Glob Chang Biol 12:272–283
Luo Z-B, Calfapietra C, Liberloo M, Scarascia-Mugnozza G, Polle A (2008) Carbon-based secondary metabolites and internal nitrogen pools in Populus nigra under Free Air CO2 Enrichment (FACE) and nitrogen fertilisation. Plant Soil 304:45–57
Matthews S, Mila I, Scalbert A, Donnelly DMX (1997) Extractable and non-extractable proanthocyanidins in barks. Phytochemistry 45(2):405–410
Mattson WJ, Julkunen-Tiitto R, Herms DA (2005) CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth-differentiation balance models? Oikos 111:337–347
McGinnis GD, Parikh S (1975) The chemical constituents of loblolly pine bark. Wood Sci 7(4):295–297
Meier D, Fortmann I, Odermatt J, Faix O (2005) Discrimination of genetically modified poplar clones by analytical pyrolysis-gas chromatography and principal component analysis. J Appl Pyrolysis 74:129–137
Mencuccini M, Hölttä T, Sevanto S, Nikinmaa E (2013) Concurrent measurements of change in the bark and xylem diameters of trees reveal a phloem-generated turgor signal. New Phytol 198:1143–1154
Natali SM, Sañudo-Wilhelmy SA, Lerdau MT (2009) Plant and soil mediation of elevated CO2 impacts on trace metals. Ecosystems 12:715–727
Norby RJ, Todd DE, Fults J, Johnson DW (2001) Allometric determination of tree growth in a CO2-enriched sweetgum stand. New Phytol 150:477–487
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG (2004) Fine-root production dominates response of deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci 101:9689–9693
Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, De Angelis P, Finzi AC, Karnosky DF, Kubiske ME, Lukac M, Pregitzer KS, Scarascia-Mugnozza GE, Schlesinger WH, Oren R (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci 102:18052–18056
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci 107:19368–19373
Overdieck D, Ziche D, Böttcher-Jungclaus K (2007) Temperature responses of growth and wood anatomy in European beech saplings grown in different carbon dioxide concentrations. Tree Physiol 27:261–268
Peterson AG, Ball JT, Luo Y, Field CB, Reich PB, Curtis PS, Griffin KL, Gunderson CA, Norby RJ, Tissue DT, Forstreuter M, Rey A, Vogel CS, Participants CMEAL (1999) The photosynthesis—leaf nitrogen relationship at ambient and elevated atmospheric carbon dioxide: a meta-analysis. Glob Chang Biol 5:331–346
Porankiewicz B, Iskra P, Sandak J, Tanaka C, Jóźwiak K (2006) High-speed steel tool wear during wood cutting in the presence of high-temperature corrosion and mineral contamination. Wood Sci Technol 40:673–682
Qiao Y-Z, Zhang Y-B, Wang K-Y, Wang Q, Tian Q-Z (2008) Growth and wood/bark properties of Abies faxoniana seedlings as affected by elevated CO2. J Integr Plant Biol 50:265–270
Raessler M, Wissuwa B, Breul A, Unger W, Grimm T (2010) Chromatographic analysis of major non-structural carbohydrates in several wood species—an analytical approach for higher accuracy of data. Anal Method 2:532–538
Rana R, Müller G, Naumann A, Polle A (2008) FTIR spectroscopy in combination with principal component analysis or cluster analysis as a tool to distinguish beech (Fagus sylvatica L.) trees grown at different sites. Holzforschung 62:530–538
Saxe H, Ellsworth DS, Heath J (1998) Tansley Review No 98 Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
So CL, Eberhardt TL (2006) Rapid analysis of inner and outer bark composition of southern yellow pine bark from industrial sources. Holz Roh Werkst 64:463–467
Spencer R, Choong ET (1977) Isolation and characterization of several extractives in the bark and wood of sweetgum. Holzforschung 31(1):25–31
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 93(2):345–354
Toscano G, Riva G, Pedretti EF, Corinaldesi F, Mengarelli C, Duca D (2013) Investigation on wood pellet quality and relationship between ash content and the most important chemical elements. Biomass Bioenergy 56:317–322
van Heerden PS, Towers GHN, Lewis NG (1996) Nitrogen metabolism in lignifying Pinus taeda cell cultures. J Biol Chem 271(21):12350–12355
Van Nevel L, Mertens J, Demey A, De Schrijver A, De Neve S, Tack FMG, Verheyen K (2014) Metal and nutrient dynamics in decomposing tree litter on a metal contaminated site. Environ Pollut 189:54–62
Warren JM, Pötzelsberger E, Wullschleger SD, Thornton PE, Hasenauer H, Norby RJ (2011) Ecohydrologic impact of reduced stomatal conductance in forest exposed to elevated CO2. Ecohydrology 4:196–210
Warren JM, Jensen AM, Medlyn BE, Norby RJ, Tissue DT (2015) CO2 stimulation of photosynthesis in Liquidambar styraciflua is not sustained during a 12-year field experiment. AoB Plants 7: plu074. doi:10.1093/aobpla/plu074
Watanabe Y, Satomura T, Sasa K, Funada R, Koike T (2010) Differential anatomical responses to elevated CO2 in saplings of four hardwood species. Plant Cell Environ 33:1101–1111
Yazaki K, Maruyama Y, Mori S, Koike T, Funada R (2005) Effects of elevated carbon dioxide concentration on wood structure and formation in trees. In: Omasa K, Nouchi I, De Kok LJ (eds) Plant responses to air pollution and global change. Springer, Tokyo, pp 89–97
Acknowledgments
This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, under contract DE-AC05-00OR22725. The authors are grateful to Fred J. Matt, USDA Forest Service, Forest Products Laboratory, for the lignin and sugar analyses; Joanne Childs and Holly Vander Stel at ORNL carried out the total phenolic content analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by T. Grams.
Rights and permissions
About this article
Cite this article
Eberhardt, T.L., Labbé, N., So, CL. et al. Effects of long-term elevated CO2 treatment on the inner and outer bark chemistry of sweetgum (Liquidambar styraciflua L.) trees. Trees 29, 1735–1747 (2015). https://doi.org/10.1007/s00468-015-1254-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1254-8