Abstract
Background
Laparoscopic technique has been increasingly used in gastrectomy, but the safety and feasibility of the laparoscopic total gastrectomy (LTG) for advanced proximal gastric cancer (PGC) after neoadjuvant chemotherapy (NAC) is unclear.
Methods
A retrospective analysis of 146 patients who received NAC followed by radical total gastrectomy at Fujian Medical University Union Hospital from January 2008 to December 2018 was performed. The primary endpoints were long-term outcomes.
Results
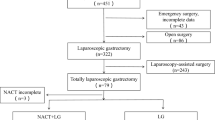
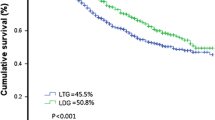
The patients were divided into two groups: 89 were in the LTG group and 57 were in the open total gastrectomy (OTG) group. The LTG group had a significantly shorter operative time (median 173 min vs. 215 min, p < 0.001), less intraoperative bleeding (62 ml vs. 135 ml, p < 0.001), higher total lymph node (LN) dissections (36 vs 31, p = 0.043), and higher total chemotherapy cycle completion rate (≥ 8 cycles) (37.1% vs. 19.7%, p = 0.027) than OTG. The 3-year overall survival (OS) of the LTG group was significantly higher than that of the OTG group (60.7% vs. 35%, p = 0.0013). Survival with inverse probability weighting(IPW) correction for Lauren type, ypTNM stage, NAC schemes and the times at which the surgery was performed showed that there was no significant difference in OS between the two groups (p = 0.463). Postoperative complications (25.8% vs. 33.3%, p = 0.215) and recurrence-free survival (RFS) (p = 0.561) between the LTG and OTG groups were also comparable.
Conclusion
In experienced gastric cancer surgery centers, LTG is recommended as the preferred option for such patients who performed NAC, owing to its long-term survival is not inferior to OTG, and it offers less intraoperative bleeding, better chemotherapy tolerance than conventional open surgery.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Wilke H, Preusser P, Fink U et al (1989) Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a Phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol 7(9):1318–1326. https://doi.org/10.1200/JCO.1989.7.9.1318
Tokunaga M, Sato Y, Nakagawa M, Aburatani T, Matsuyama T, Nakajima Y et al (2020) Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today 50:30–7
Ajani Jaffer A, D’Amico Thomas A, Bentrem David J (2022) Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(2):167–192
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Katai H, Mizusawa J, Katayama H et al (2020) Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 5(2):142–151
Kim HH, Han SU, Kim MC, Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group et al (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 5(4):506–513. https://doi.org/10.1001/jamaoncol.2018.6727
Huang CM, Liu H, YFHu et al (2022) Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg 157(1):9–17
Li Z, Shan F, Ying X et al (2019) Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg 154(12):1093–101
An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH (2012) Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol 19(8):2452–8
Fujisaki M, Mitsumori N, Shinohara T et al (2020) Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc 35:1682–1690
van der Wielen N, Straatman J, Daams F (2021) Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer 24(1):258–271
Amin MB, Edge S, Greene F et al (eds) (2017) 8th edn. Springer, New York
Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, Muro K et al (2010) Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer(G-SOX study). Ann Oncol 21:1001–1005
Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH et al (2014) Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 140:319–328
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S et al (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92:1644–9
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma 3rd English edition. Gastric Cancer 14:101–12
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123. https://doi.org/10.1007/s10120-011-0042-4
JGC Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver 4). Gastric Cancer 20(1):1–19
De Steur WO et al (2015) Quality control of LN dissection in the Dutch gastric cancer trial. Br J Surg 102:1388–1393
Chen QY et al (2019) Laparoscopic total gastrectomy for upper-middle advanced gastric cancer: analysis based on LN noncompliance. Gastric Cancer 23:184–194
Dindo D et al (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205
Busweiler LA, Schouwenburg MG, van Berge Henegouwen MI, Kolfschoten NE, de Jong PC, Rozema T et al (2017) Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg 104(6):742–750
Bin-Bin Xu, Jun Lu, Zheng Zhi-Fang (2019) The predictive value of the preoperative C-reactive protein-albumin ratio for early recurrence and chemotherapy benefit in patients with gastric cancer after radical gastrectomy: using randomized phase III trial data. Gastric Cancer 22(5):1016–1028
Cole R Stephen, Hernán Miguel A (2004) Adjusted survival curves with inverse probability weights. Comput Methods Progr Biomed 75(1):45–49
Fujisaki M, Mitsumori N (2021) Toshihiko, ShinoharaShort-and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc 35(4):1682–1690
Bin-Bin Xu, Jun Lu, Zheng Zhi-Fang (2019) Comparison of short-term and long-term efficacy of laparoscopic and open gastrectomy in high-risk patients with gastric cancer: a propensity score-matching analysis. Surg Endosc 33(1):58–70
Liu F-L, Huang C-M, Xu Ze-kuan (2020) Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: The CLASS02 multicenter randomized clinical trial. JAMA Oncol 6(10):1590–1597
Al-Batran SE, Homann N, Pauligk C et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393:1948–1957
Funding
Fujian Province Medical "Creating Double High" Construction Funding Support (Fujian Health Medical Administration NO.[2021]76). Funder: Huang Chang-ming.
Author information
Authors and Affiliations
Contributions
LS, HLZ and BX contributed equally to this work and should be considered first coauthors. CHZ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: LS, CHZ. Acquisition, analysis, or interpretation of data: JL, LS, QYC, ZX, BX, PL, JL. Drafting of the manuscript: LS, HLZ, BX. Critical revision of the manuscript for important intellectual content: HLZ, CHZ, LS, JWX, CMH. Statistical analysis: LS. Administrative, technical, or material support: CHZ. Supervision: CHZ.
Corresponding author
Ethics declarations
Disclosures
Drs. Hua-Long Zheng, Li–li Shen, Bin-bin Xu, Qi-Yue Chen, Jun Lu, Zhen Xue, Jia-Lin, Jian-Wei **e, ** Li, Chang-Ming Huang, Chao-Hui Zheng have no conflicts of interest to disclose.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Consent for publication
This article doesn’t report an individual participant's data in any form.
Validity and feasibility of the statistics
The validity and feasibility of statistics have been evaluated by a biostatistician, Wu FQ.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


464_2023_10084_MOESM3_ESM.jpg
Supplementary file3 (JPG 1194 KB)—eFig. 2 Comparison of LN dissections between laparoscopic total gastrectomy (LTG) group and open total gastrectomy (OTG) group. A Comparison of mean No. of lymph node dissection in each station between the two groups. B, C Comparison of mean No. of lymph node dissection in each station between the two groups according to RECIST standard. B CR/PR subgroup. C PD/SD subgroup. D, E Comparison of mean No. of lymph node dissection in each station between the two groups according to ypTNM stage. D stage I. E stage II/III
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, HL., Shen, Ll., Xu, Bb. et al. Oncological outcomes of laparoscopic versus open radical total gastrectomy for upper-middle gastric cancer after neoadjuvant chemotherapy: a study of real-world data. Surg Endosc 37, 6288–6297 (2023). https://doi.org/10.1007/s00464-023-10084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10084-z