Abstract
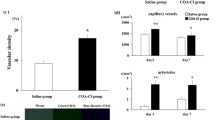
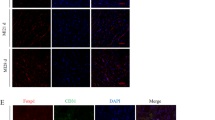
Isosteviol has been indicated as a cardiomyocyte protector. However, the underlying mechanism remains unclear. Thus, we sought to confirm the protective effect of isosteviol after myocardial infarction in a model of permanent coronary artery occlusion and investigate the potential proangiogenic activity in vitro and in vivo. A 4-week permanent coronary artery occlusion rat model was generated, and the protective effect of isosteviol was evaluated by echocardiographic imaging and hemodynamics assays. The coronary capillary density was tested by immunochemistry and micro-computed tomography (μCT) imaging. The effect of isosteviol on endothelial cells was determined in human umbilical vein endothelial cells (HUVECs) in vitro and Tg (kdrl: EGFP) zebrafish in vivo. We also examined the expression of related transcription factors by real-time polymerase chain reaction (RT-qPCR). Isosteviol increased ejection fraction (EF), fractional shortening (FS), cardiac systolic index (CI), maximum rate of increase of left ventricular pressure (Max dp/dt), and left ventricular systolic pressure (LVSP) by 32%, 40%, 25%, 26%, and 10%, respectively, in permanent coronary artery occlusion rats. Interestingly, it also promoted coronary capillary density by 2.5-fold. In addition, isosteviol promoted the proliferation and branching of HUVECs in vitro. It also rescued intersegmental vessel (ISV) development and improved endothelial cell proliferation by approximately fivefold (4–6) in zebrafish embryos in vivo. Isosteviol also upregulated the expression of hypoxia inducible factor-1α (HIF-1α) and vascular endothelial growth factor A (VEGFA) in zebrafish by fourfold and 3.5-fold, respectively. Our findings suggest that isosteviol is a proangiogenic agent and that this activity is related to its protective effects against myocardial ischemia.
Graphical abstract
After using the permanent coronary artery occlusion model, we demonstrated that isosteviol promotes angiogenesis directly and increases capillary density in myocardial ischemia rats. Isosteviol promotes angiogenesis in zebrafish in vivo and increases vascular endothelial cell proliferation in HUVECs and zebrafish. The angiogenesis activity of isosteviol may be correlated with VEGFA and HIF-1α signaling.







Similar content being viewed by others
References
Albrecht-Schgoer K, Schgoer W, Theurl M et al (2014) Topical secretoneurin gene therapy accelerates diabetic wound healing by interaction between heparan-sulfate proteoglycans and basic FGF. Angiogenesis 17(1):27–36
Beis D, Bartman T, ** SW et al (2005) Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132(18):4193–4204
Chen Y, Beng H, Su H et al (2019) Isosteviol prevents the development of isoprenaline induced myocardial hypertrophy. Int J Mol Med 44(5):1932–1942
De Vries M, Seppala LJ, Daams JG et al (2018) Fall-risk-increasing drugs: a systematic review and meta-analysis: I. Cardiovascular drugs. J Am Med Dir Assoc 19(4), 371:1–9
Eibel B, Markoski MM, Rodrigues CG et al (2017) VEGF Gene Therapy Cooperatively Recruits Molecules from the Immune System and Stimulates Cell Homing and Angiogenesis in Refractory Angina. Cytokine 91:44–50
Fan Z, Wen T, Chen Y et al (2016) Isosteviol sensitizes sarcK(ATP) channels towards Pinacidil and potentiates mitochondrial uncoupling of diazoxide in guinea pig ventricular myocytes. Oxid Med Cell Longev 2016:6362812
Fu X, Ou B (2020) miR-152/LIN28B axis modulates high-glucose-induced angiogenesis in human retinal endothelial cells via VEGF signaling. J Cell Biochem 121(2):954–962
Garcia-Prieto J, Villena-Gutierrez R, Gomez M et al (2017) Neutrophil stunning by metoprolol reduces infarct size. Nat Commun 4(8):14780
Goodwill AG, Dick GM, Kiel AM et al (2007) Regulation of coronary blood flow. Compr Physiol 7(2):321–382
Hashimoto H, Olson EN, Bassel-Duby R (2018) Therapeutic approaches for cardiac regeneration and repair. Nat Rev Cardiol 15(10):585–600
Huang H, Lindgren A, Wu X et al (2012) High-throughput screening for bioactive molecules using primary cell culture of transgenic zebrafish embryos. Cell Rep 2(3):695–704
Jiang L, Zeng H, Ni L et al (2019) HIF-1α preconditioning potentiates antioxidant activity in ischemic injury: the role of sequential administration of dihydrotanshinone I and protocatechuic aldehyde in cardioprotection. Antioxid Redox Signal 31(3):227–242
Ke Q, Liu F, Tang Y et al (2021) The protective effect of isosteviol sodiumon cardiac function and myocardial remodeling in transverse aortic constriction rat. J Cell Mol Med 25(2):1166–1177
Kivela R, Bry M, Robciuc MR et al (2014) VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Mol Med 6(3):307–321
Lazarous DF, Shou M, Scheinowitz M et al (1996) Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation 94(5):1074–1082
Lederman RJ, Mendelsohn FO, Anderson RD et al (2002) Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomized trial. Lancet 359(9323):2053–2058
Li H, Zhou Y, Li L et al (2019) HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol vol. 25 101109
Liu F, Su H, Liu B et al (2020) STVNa attenuates isoproterenol-induced cardiac hypertrophy response through the HDAC4 and Prdx2/ROS/Trx1 pathways. Int J Mol Sci 21:6822
Liu N, Wu M, Chen C et al (2009) Novel molecular targets participating in myocardial ischemia-reperfusion injury and cardioprotection. Cardiol Res Pract 2019:6935147
Liu Y, Asnani A, Zou L et al (2014) Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase. Sci Transl Med 6:70266
Mei Y, Liu B, Su H et al (2020) Isosteviol sodium protects the cardiomyocyte response associated with the SIRT1/PGC-1 alpha pathway. J Cell Mol Med 24(18):10866–10875
Melincovici CS, Bosca AB, Susman S et al (2018) Vascular endothelial growth factor (VEGF) — key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2:455–467
Mitsos S, Katsanos K, Koletsis E et al (2012) Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials. Angiogenesis 15(1):1–22
Muenzel T, Daiber A (2018) Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vascul Pharmacol 102:1–10
Nowak-Sliwinska P, Alitalo K, Allen E et al (2018) Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21(3):425–532
Pagliaro BR, Cannata F, Stefanini GG et al (2020) Myocardial ischemia and coronary disease in heart failure. Heart Fail Rev 25(1SI):53–65
Risau W (1997) Mechanisms of angiogenesis. Nature 386(6626):671–674
Serocki M, Bartoszewska S, Janaszak-Jasiecka A et al (2018) MiRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis 21(2):183–202
Sun X, Yang Y, **e Y et al (2018) Protective role of STVNa in myocardial ischemia reperfusion injury by inhibiting mitochondrial fission. Oncotarget 9(2):1898–1905
Tang S, Liu X, Ye J et al (2018) Isosteviol ameliorates diabetic cardiomyopathy in rats by inhibiting ERK and NF-kappa B signaling pathways. J Endocrinol 238(1):47–60
Timar J, Dome B, Fazekas K et al (2001) Angiogenesis-dependent diseases and angiogenesis therapy. Pathol Oncol Res: POR 7(2):85–94
Toldo S, Mauro AG, Marchetti C et al (2016) Recombinant HumanAlpha-1 Antitrypsin-Fc fusion protein reduces mouse myocardial inflammatory injury after ischemia-reperfusion independent of elastase inhibition. J Cardiovasc Pharmacol 68(1):27–32
Yang Y, Shi C, Hou X et al (2015) Modified VEGF targets the ischemic myocardium and promotes functional recovery after myocardial infarction. J Control Release 213:27–35
Yang Y, Zhong Q, Zhang H et al (2018) Lipidomics study of the protective effects of isosteviol sodium on stroke rats using ultra high-performance supercritical fluid chromatography coupling with ion-trap and time-of-flight tandem mass spectrometry. J Pharm Biomed Anal 157:145–155
Yuan R, **n Q, Shi W et al (2018) Vascular endothelial growth factor gene transfer therapy for coronary artery disease: a systematic review and meta-analysis. Cardiovasc Ther 36:e124615
Zhang H, Liu B, Xu G et al (2019) Synthesis and in vivo screening of isosteviol derivatives as new cardioprotective agents. Eur J Med Chem vol. 219 2021:113396
Zhao H, Osborne OJ, Lin S et al (2016) Lanthanide hydroxide nanoparticles induce angiogenesis via ROS-sensitive signaling. Small 12(32):4404–4411
Acknowledgements
We thank Dr. Khaja Shameem Mohammed Abdul and Dr. Shan** Wang for his assistance with the language editing in the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81903600 and No.81802123), China Postdoctoral Science Foundation (Grant No. 2018M643026, the Science and Technology Innovation Project of Foshan (Grant No. 2017IT100162), and Technology Major Projects for “Major New Drugs Innovation and Development (2019ZX09301120).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in the studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of Guangdong Pharmaceutical University (Approval No. 20180714–03).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
441_2021_3559_MOESM1_ESM.tif
Supplementary file1 Fig. 1 The embryos of wild type and Tg (kdrl: EGFP) zebrafish were used for gating in flow cytometry. (a) The embryos of wild type zebrafish were used as unstained control. P2 was gated based on the population in the unstained control (0.3%, close to 0%). (b) The gating for Tg (kdrl: EGFP) zebrafish groups was determined by referring to the unstained control. The representative image of isosteviol shown that the fluorescent intensity increased (7%) (TIF 1721 KB)
441_2021_3559_MOESM2_ESM.tif
Supplementary file2 Fig. 2 The left ventricular wall thickness and dimension had no significant difference after treatment with isosteviol. (a) Left ventricular end-diastolic posterior wall thickness (LVPW;d) had no significant difference after treatment with isosteviol. n = 8–10 per group. (b) Left ventricular end-systolic posterior wall thickness (LVPW;s) had no significant change after treatment with isosteviol. n = 8–10 per group. (c) Left ventricular end-diastolic internal dimension (LVID;d) had no significant difference after treatment with isosteviol. n = 8–10 per group; (d) Left ventricular end-systolic internal dimension (LVID;s) decreased in the isosteviol group compared with the model group but had no significant difference. #P < 0.05, vs sham group n = 8–10 per group (TIF 3786 KB)
441_2021_3559_MOESM3_ESM.tif
Supplementary file3 Fig. 3 The energy statue had no significant difference after treatment with isosteviol. (a) Respiratory quotient (RQ) was similar in all groups; (b) Isosteviol moderately rescued EE compared with model group but had no significant difference. n = 8–10 per group (TIF 1964 KB)
Rights and permissions
About this article
Cite this article
Liu, F., Song, L., Lu, Z. et al. Isosteviol improves cardiac function and promotes angiogenesis after myocardial infarction in rats. Cell Tissue Res 387, 275–285 (2022). https://doi.org/10.1007/s00441-021-03559-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03559-9