Abstract
Purpose
We designed this study to evaluate the impact of intraoperative intravenous lidocaine infusion on postoperative opioid consumption after laparoscopic cholecystectomy.
Methods
In total, 98 patients scheduled for elective laparoscopic cholecystectomy were included and randomized. In the experimental group, intravenous lidocaine (bolus 1.5 mg/kg and continuous infusion 2 mg/kg/h) was administered intraoperatively additionally to the standard analgesia, whereas the control group received a matching placebo. Blinding existed at the level of both the patient and the investigator.
Results
Our study failed to confirm any benefit in opioid consumption, during the postoperative period. Lidocaine resulted to reduced intraoperative systolic, diastolic, and mean arterial pressure. Lidocaine administration did not change postoperative pain scores or the incidence of shoulder pain, at any time endpoint. Moreover, we did not identify any difference in terms of postoperative sedation levels and nausea rates.
Conclusion
Overall, lidocaine did not have any effect on postoperative analgesia after laparoscopic cholecystectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rationale
Cholecystectomy is among the most commonly performed procedures worldwide [1]. The implementation of minimal invasive principles during the previous decades resulted in improved perioperative outcomes, such as postoperative pain, morbidity, quality of recovery, and cosmesis [2, 3]. These benefits, combined with a relatively short learning curve [4], enabled an early and widespread adoption of laparoscopic cholecystectomy.
Even though laparoscopic cholecystectomy is currently the gold standard for the management of most elective and emergency gallstone disease, perioperative analgesia still remains an important clinical issue [5,6,7]. More specifically, a considerable proportion of patients, in some cases up to 50% [8], complain about postoperative pain. Among the reported symptoms are superficial incision pain, abdominal pain, and post-laparoscopy shoulder pain [8, 9]. Suboptimal postoperative pain control after laparoscopic cholecystectomy results to prolonged hospitalization and worsening quality of recovery, thus inhibiting the effective implementation of a day-surgery policy [10, 11].
Therefore, to enhance postoperative pain management and reduce opioid consumption, multimodal analgesia protocols were introduced. These protocols consist of multiple synergetic interventions and medications, such as patient-controlled analgesia, local anesthetics, and non-steroidal anti-inflammatory drugs [10, 12]. Administration of intravenous lidocaine is another strategy whose role has been recently studied in abdominal surgery, with promising results [13,14,15]. Besides the potent analgesic and anti-inflammatory effect of intravenous lidocaine, there are also several other properties of this drug that accelerate postoperative recovery [14].
Current evidence regarding the role of intravenous lidocaine infusion in laparoscopic cholecystectomy is conflicting. Initial reports proposed a significant analgetic and opioid-sparing effect that resulted to reduced morbidity and shortened length of hospital stay [7, 10, 12, 16]. These findings were further validated by two successive meta-analyses [10, 12]. However, these pooled results were based on small size studies and heterogeneous lidocaine dosages. Moreover, recent clinical data questioned the analgesic role of intravenous lidocaine, thus adding to the overall discrepancy [11, 16].
Objectives
We designed and conducted the present study to elucidate the impact of intravenous lidocaine infusion on postoperative opioid consumption and pain intensity after laparoscopic cholecystectomy.
Methods
Design
This study was designed as a prospective randomized controlled trial (RCT) and conducted at the University Hospital of Larissa. A local ethics committee approval was obtained prior to the onset of the trial (UHL2782 /06/28/2018). The protocol record was, also, enlisted in an electronic registry (ClinicalTrials.gov Identifier: NCT03620591). All eligible patients provided a signed informed consent. The present study report adheres to the CONSORT statement guidelines [17].
Participants
All consecutive adult patients (age ≥ 18 years) scheduled for an elective laparoscopic cholecystectomy during the recruitment period, with an American Society of Anesthesiologists (ASA) score I or II, and a body mass index (BMI) ≤ 35, were considered eligible. The following exclusion criteria were included: (1) age < 18 years, (2) ASA physical status ≥ III, (3) patient’s refusal to provide a signed informed consent, (4) allergy in local anesthetics, (5) medical history of liver, renal, or heart failure, (6) chronic pain or (7) systematic analgesics consumption, (8) psychiatric disorders, and (9) inability to perceive and evaluate postoperative pain levels.
Interventions
After the outpatient pre-anesthetic evaluation, all eligible patients were submitted to an elective laparoscopic cholecystectomy. The numeric rate scale (NRS) for pain at rest (NRS rest) and at stress (NRS stress) was explained to patients (0 = no pain, 10 = the worst imaginable pain) during their evaluation. On patients’ arrival in the operating room, non-invasive monitoring (ECG, NIBP, SPO2) was established. Patients were monitoring continuously intraoperatively, and their vital signs (systolic, diastolic, and mean arterial pressure, heart rate) were recorded at a 5-min interval. Prior to anesthesia onset, 1 mg midazolam, 40 mg ondansetron, and 50 mg ranitidine were administered. In the experimental group, patients received a bolus (1.5 mg/kg) lidocaine dose, followed by a continuous lidocaine infusion (2 mg/kg/h), until the procedure end. Control patients received a corresponding placebo infusion (normal saline 0.9%). To avoid intraoperative adverse events, standard safety protocols were utilized. To be more specific, a decrease in the mean arterial pressure > 20% of the base value was managed with the bolus administration of 50 mcg i.v. phenylephrine.
Introduction to anesthesia included the administration of propofol (2 mg/kg), fentanyl (3 μg/kg), and rocuronium bromide (0.6 mg/kg). Anesthesia maintenance was based on a desflurane air/O2 mixture with a 40 to 60 bispectral index (BIS). Neuromuscular blockade was reversed with sugammadex. Extubation was performed when patients could obey simple demands. Intraoperatively, all patients received paracetamol 1 g and dexamethasone 8 mg. Post-anesthesia care unit discharge was based on the Aldrete score [18].
Surgical approach was based on the standard 4-port laparoscopic technique. Following the open Hasson technique, pneumoperitoneum was set at 12 mmHg. Intraoperatively, the patients were placed in a reverse Trendelenburg, combined with a left-sided tilt, position. Prior to trocar placement, local wound infiltration (ropivacaine solution, 0.375%, 20 mL) was performed.
Postoperative care was standardized for all patients and included the administration of crystalloids, thromboprophylaxis (20 mg enoxaparin sodium), and the early mobilization and onset of diet. Standard analgesia consisted of paracetamol every 8 h. In case of increased pain levels (NRS > 3), supplementary opioid analgesia was administered (tramadol 100 mg i.v.). All patients were discharged at 24 h, provided the fulfillment of the Clinical Discharge Criteria [19].
Outcomes
The primary outcome of this study was the difference between the two groups in terms of opioid consumption (total tramadol dose) at 24 h postoperatively. Secondary outcomes included tramadol requirements at various time endpoints 0 (discharge of post care anesthesia unit), 6, 12, and 24 postoperative h. Pain evaluation included NRS measurements at rest and during movement at the same time intervals. Similarly, the incidence of headache and shoulder pain was assessed. Sedation level was appraised by the Ramsay scale [20], while postoperative nausea and vomiting were graded by a 4-point Likert scale (0 = nil, 1 = mild nausea, 2 = moderate nausea, 3 = severe nausea and vomiting).
Randomization and blinding
A specialized electronic software was used for the 1:1 parallel randomization process. The allocation group masking included the use of sealed opaque envelopes. Blinding existed at the level of the patient and the independent third-party investigator, responsible for data recording.
Statistical analysis
Sample size estimation was based on the primary endpoint. In a pilot sample of 10 control laparoscopic cholecystectomies, the mean tramadol dose at 24 h was 150 (57.7) mg. Therefore, assuming a 25% decrease in the opioids consumption in the experimental group, with a 5% alpha, 90% power, and a 5% dropout rate, the total estimated sample size was 98 patients.
A per-protocol analysis was implemented. Prior to any analyses, all data underwent a Shapiro–Wilk normality test. Categorical variables were compared by a Pearson chi square test. An independent Student t-test was used for the comparison of normal continuous outcomes. In non-normal variables, a Mann–Whitney test was introduced. Correlation between continuous variables was assessed by Spearman’s rank-order test. Multiple group comparisons were performed by the Kruskal–Wallis test, while ad hoc analyses were based on the Mann–Whitney test. The Friedman test, followed by the Wilcoxon signed-rank test, was introduced for the evaluation of time-dependent pairwise comparisons of continuous data. Correspondingly, repeated measures of categorical variables were evaluated by the Cochran Q test.
The acceptable percentage of missing data was < 10% [21]. Missing values were managed through the implementation of the multiple imputation method. Continuous and categorical variables were presented as mean (standard deviation (SD)) and N (percentage), respectively. In non-normal distributions, continuous outcomes were provided as median (interquartile range (IQR)). Statistical significance was considered at the level of p < 0.05. All analyses were performed in the IBM SPSS Statistics v23 software.
Results
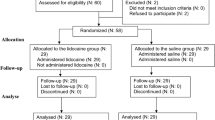
Due to the COVID-19 pandemic, all elective operations and outpatient departments were halted during the 2020–2021 period. Following a steering committee convention, the study recruitment period was extended until the completion of the estimated sample size. Overall, 126 patients (Fig. 1) were screened for eligibility during the study accrual period (July 2018–January 2022). From these, 19 cases failed to fulfill the inclusion criteria, while 9 patients refused to participate in the study. Therefore, 98 patients were eventually included and randomized to the study subgroups. There was no conversion to open, and no patient was lost to follow-up. Therefore, all cases were submitted to statistical analysis.
Table 1 summarizes the characteristics of the included patients. There was no significant difference in demographics between the two groups. In total, 37 males and 61 females (p = 0.835) were submitted to laparoscopic cholecystectomy. Most patients had mild comorbidities (ASA I: 35.7%, ASA II: 64.3%, p = 0.527). The two groups received an equivalent intraoperative dose of fentanyl (p = 0.107). Similarly, operation duration was comparable between the experimental and the control arm (50.6 vs 50 min, p = 0.319). Interestingly, the lidocaine subgroup was associated with a significantly prolonged extubation period (7 vs 6 min, p = 0.018). Further explanatory analyses (Supplementary Material Tables) confirmed that extubation duration was associated only with the allocation group.
The administration of lidocaine resulted in a statistically significant intraoperative reduction of systolic, diastolic, and mean arterial pressure, after the induction of anesthesia (Fig. 2; Supplementary Material Tables). The mean heart rate, though, was not affected. Similar results were estimated when the set time point was the introduction of pneumoperitoneum (Fig. 3; Supplementary Material Tables).
Regarding the primary outcome (Table 2), the administration of lidocaine did not affect the opioid requirements at 24 h, postoperatively (p = 0.542). Similar results were recorded at any time endpoint evaluated (tramadol dose at 0 h p = 0.391, at 6 h p = 0.767, at 12 h p = 0.296).
Postoperative pain at rest measurements (Table 2; Supplementary Material Figures) displayed a declining trend throughout the postoperative period in both groups (p < 0.001). More specifically, median NRS scores decreased from 3 at 0 h to 2 at 24 h. Pairwise comparisons could not identify any significant impact of lidocaine administration on pain scores, at any time endpoint evaluated. In addition to these, pain measurements at movement were, consistently, not affected by the allocation group.
A small number of the entire cohort of patients (14.3%) reported shoulder pain symptoms at 12 h postoperatively. The incidence of these symptoms was further decreased at discharge (24 h: 5.1%). Nonetheless, group allocation was not associated with shoulder pain rates.
Postoperative headache was recorded only in two cases in the lidocaine group (at 12 and at 24 h). No patient developed nausea or vomiting. Sedation was consistent throughout the postoperative period, with no variation, and all patients were co-operative, oriented, and tranquil.
Discussion
Summary of evidence
Lidocaine was first introduced in the early 50 s [22]. It is an amino-amide type local anesthetic, whose main mechanism of action is the blocking of voltage-gated sodium channels (VGSCs) [23]. More specifically, the free-base molecule passes through the lipophilic cellular membrane and binds to the segment 4 of domain 6 of the a-subunit of the ion channel, thus inhibiting the sodium influx and the action potential propagation [23]. The intravenous administration of lidocaine, though, may involve more complex biochemical interactions [24]. Animal studies suggested that in neuropathic pain, lidocaine binds to specific ion channels in the dorsal root ganglia and inhibits the activation of the respective nerves [24]. However, in patients with normal pain thresholds, the exact pathway of lidocaine is vaguer [22,23,24,25,26]. Among the cellular targets that have been examined are membrane receptors, modulation of K + and Ca2 + channels, N-methyl-d-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-4-izoxazolepropionicacid (AMPA) receptors, and GTP-binding protein coupling receptors [22,23,24,25,26]. In a recent in vivo study, Kurabe et al. [24] proposed a glutamate-inhibition effect of intravenous lidocaine at the presynaptic terminals that results to a membrane potential shift and a post-synaptic hyperpolarization [24].
Initially, the role of lidocaine as part of a multimodal approach was examined in major operations, where analgesia requirements are increased [27]. In the study by Koppert et al. [27], patients who underwent major abdominal surgery received IV lidocaine until the 1st postoperative hour. The experimental group displayed significantly lower pain scores and morphine consumption during the first 72 h [27]. Subsequent pooled analyses further validated these findings, thus suggesting a potent analgesic effect of lidocaine [28, 29]. However, a recent Cochrane meta-analysis pointed out to a limited and early-phase benefit that does not extend to the subsequent postoperative days [15]. This discrepancy may be explained by the use different pain assessment tools, a wide range of lidocaine dosing regimens, and the extent of the operation [15]. Regarding the latter, the implementation of a minimal invasive approach decreases tissue injury, thus reducing the clinical impact of lidocaine [30, 31]. As shown by our research group, although lidocaine abates pain scores and opioid consumption in open colorectal resections [30], this is not the case in the laparoscopic setting [31].
Laparoscopic cholecystectomy is performed through a standard four-port technique. Despite its benefits, the increased incidence of postoperative pain impedes the implementation of an efficient recovery protocol. Post-laparoscopic cholecystectomy pain includes three distinct subtypes [9]. First, tissue trauma in the port insertion sites results to somatic pain [9]. Moreover, intraoperative tissue handling combined with pneumoperitoneum-related diaphragmatic irritation is responsible for the development of visceral pain. Finally, abdominal distention and CO2-induced peritoneal inflammation that leads to arteriole tearing and nerve fiber injury promote phrenic nerve neuropraxia and, subsequently, the emergence of shoulder pain [32]. Visceral pain is the main form during the first postoperative day; on the contrary, shoulder-tip pain symptoms may exist up to 72 h, postoperatively [9].
Numerous studies attempted to quantify the clinical benefit deriving from the intravenous administration of lidocaine during laparoscopic cholecystectomy. In a RCT by Song et al. [7], intraoperative lidocaine infusion, when compared to placebo, decreased mean visual analog scores at 2 and 6 h after surgery. Two recent meta-analyses by Zhao et al. [10] and Li et al. [12] indicated a significant analgesic effect of lidocaine that extended up to the second postoperative day. In parallel with the subjective pain measurements, the respective opioid consumption was considerably reduced over the control subgroup [10, 12]. On the contrary, Ortiz et al. [16] reported that lidocaine infusion did not alter postoperative pain scores at rest and coughing and total morphine consumption. Our results are similar; NRS scores at rest and movement were comparable at all endpoints. Additionally, contrary to our initial study assumption, overall tramadol consumption did not decrease in the experimental group.
An important factor that should be commented on is the potential effect of local wound infiltrations on the overall pain outcomes. In our study, both subgroups received ropivacaine infiltrations in the port incisions, thus limiting any bias. Theoretically, local infiltrations may affect somatic pain, but not visceral or shoulder-tip pain. Although local anesthetic infiltration may act as a confounder in early pain measurements, it is a common practice in multiple large-scale RCTs in the field of minimal invasive surgery [33, 34]. Moreover, current evidence regarding the exact analgesic effect of local infiltrations is still inconclusive [35,36,37].
Besides analgesia, lidocaine displays multiple other properties, including anti-hyperalgesia, anti-allodynia, and anti-inflammatory effects [23]. Regarding the latter, it was shown that lidocaine inhibits the priming of human polymorphonuclear cells or neutrophils and inhibits the release of inflammatory cytokines and chemokines [23]. More specifically, Sridhar et al. [38] validated a significant reduction in postoperative total leukocyte count and circulating CRP and interleukin (IL)-6 levels after IV lidocaine infusion. Similarly, Ortiz et al. [16] showed that lidocaine reduces serum levels of IL-1, IL-6, interferon-γ, and tumor necrosis factor-a during laparoscopic cholecystectomy. These anti-inflammatory effects did not have an effect, though, on bowel function recovery or length of hospital stay [16]. On the contrary, Song et al. [7] reported a shorter time to first flatus and time to first bowel movement when lidocaine was administered. In our protocol, we did not look specifically at those parameters as all patients received per os diet and were discharged during the first 24 h on a day case basis.
Opioids are commonly used for perioperative pain management [39]. Intraoperatively, opioids minimize pain symptoms and inhibit the sympathetic response, thus facilitating anesthesia maintenance [39]. During the postoperative period, opioids modulate pain signal transmission and the response to painful stimuli, thus enabling efficient pain control [39]. However, opioids are associated with multiple side effects, such as nausea, vomiting, and respiratory depression [39]. Besides these, prolonged hospitalization and the development of addiction impact postoperative recovery, especially under the light of the recent opioid over-prescription crisis [39]. Thus, multimodal analgesia has been proposed as a mean to withhold opioid administration [39, 40]. Several studies confirmed the opioid-sparing effect of intravenous lidocaine in laparoscopic cholecystectomy [10] and the respective improvement on the quality of postoperative recovery [11]. As mentioned earlier, we estimated similar tramadol requirements in the lidocaine and the control group. Respectively, there was no difference in the nausea and vomiting rates at all estimated time endpoints.
The stabilization of the intraoperative hemodynamic variables is a significant parameter that affects the perioperative mortality and morbidity [41]. More specifically, intubation and introduction of pneumoperitoneum result to notable cardiovascular fluctuations that derive from the activation of the sympathetic nervous system [41]. Therefore, the optimal drug should inhibit the excretion of catecholamines, without affecting the cerebral blood flow and the efficacy of the anesthetic regimen [41]. Lidocaine has exhibited these properties in multiple clinical scenarios, where a significant decrease in mean arterial pressure and cardiac index was found [41,42,43]. However, these were not confirmed in the study by Lu et al. [11]. Our RCT validated a significant reduction of systolic, diastolic, and mean arterial pressure measurements in the lidocaine group, regardless of the time set point. No effect in the mean heart rate was found, though.
Despite these, the development of intraoperative hypotension may result to organ ischemia and, therefore, suboptimal postoperative outcomes [44]. More specifically, in a large multicenter cohort of 368,222, non-cardiac procedures showed that the risk for a major cardiac or cerebrovascular adverse event in the 30-day postoperative follow-up period was 12% with mean arterial pressure ≤ 75 mmHg [44]. In cases of sever hypotension (≤ 55 mmHg), that risk increased to 26% [44]. In our study, a safety protocol was applied, where a reduction of over 20% of the base mean arterial pressure was managed with the administration of bolus phenylephrine. In addition to these, mean MAP values were systematically retained over the 75 mmHg threshold in both study subgroups.
In our cohort, a significant delay in the time to extubation (7 versus 6 min) was noted in the experimental subgroup. This finding is in parallel with the current literature. In a recent RCT by Dogan et al. [45], it was shown that compared to esmolol, lidocaine infusion was superior in terms of tracheal responses with a longer time to extubation, though. Similar results were provided by the meta-analysis by Yang et al. [46]; time to extubation was significantly longer when lidocaine was administered compared to placebo. The authors acknowledged the role of volatile agents and confirmed the effect of halothane, but not that of sevoflurane and isoflurane [46]. We applied a desflurane-based anesthesia maintenance which is a favorable agent, with minimal extubation periods [47, 48]. Moreover, to reduce the confounding of the administered agent to the clinical outcomes, we targeted the depth of anesthesia to pre-defined BIS measurements.
Patient safety is another major issue that should be addressed when evaluating a novel analgesic strategy. Intravenous lidocaine is associated with a very narrow therapeutic range, with early toxicity. Previous reports estimated that serious adverse events occur beyond the level of 9–10 μg/mL [26, 49]. Although initial toxicity may present with neuropsychiatric symptoms, such as light headedness, tinnitus, altered speech, and muscle twitching, higher plasma levels result to loss of consciousness, myocardial depression, and, ultimately, cardiac and respiratory arrest [26]. It must be noted, though, that factors that affect bioavailability and clearance (e.g., distribution volume, cardiac index, hepatic and renal impairment, acid–base status, hypoalbuminemia, and medications) alter the correlation between dosage and toxicity [26]. Large cohort analyses reported a minimal minor adverse event rate (6.8%), thus confirming the safety of lidocaine [26]. Moreover, a recent pooled analysis of 2802 cases [50] concluded that the administration of lidocaine did not result to an increased risk of agent-related complications. Although cardiac adverse events were reported, most of them were sporadic and unrelated to lidocaine [50]. Similarly, we did not record any mild or severe adverse event in the experimental arm.
Limitations
Prior to the appraisal of our results, several limitations should be acknowledged. First, despite the introduction of an adequate randomization and blinding algorithm, the inherent heterogeneity in patient characteristics may have affected the final outcomes of our trial. Moreover, a larger patient pool may have affected comparative results, although the estimated sample size was based on previous observations and adequate power. More specifically, failure to confirm data normality prohibited the performance of further explanatory and correlation analyses. Furthermore, despite the use of validated scales [20], the subjective nature of several, patient-assessed, secondary outcomes represented an additional source of bias. Additionally, based on the study protocol, evaluation of inflammatory markers, hospitalization duration, and bowel function recovery was not performed, thus narrowing the measured effect of lidocaine in the postoperative period. Finally, the intraoperative infiltration of local anesthetic may have had a confounding effect on the postoperative pain measurements.
Conclusions
Our study failed to confirm the presence of any benefit of lidocaine, in terms of opioid consumption, during the postoperative period after laparoscopic cholecystectomy. Additionally, lidocaine administration did not affect postoperative pain scores or the incidence of shoulder pain, at any time endpoint evaluated. Moreover, we did not identify any difference in terms of postoperative sedation levels and nausea rates. Due to the abovementioned study limitations, further RCTs with larger sample size are required to validate our results.
References
Abbott TEF, Fowler AJ, Dobbs TD et al (2017) Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth 119:249–257. https://doi.org/10.1093/bja/aex137
Zhao JJ, Syn NL, Chong C et al (2021) Comparative outcomes of needlescopic, single-incision laparoscopic, standard laparoscopic, mini-laparotomy, and open cholecystectomy: a systematic review and network meta-analysis of 96 randomized controlled trials with 11,083 patients. Surgery 170:994–1003. https://doi.org/10.1016/j.surg.2021.04.004
Coccolini F, Catena F, Pisano M et al (2015) Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg 18:196–204. https://doi.org/10.1016/j.ijsu.2015.04.083
Reitano E, DeAngelis N, Schembari E et al (2021) Learning curve for laparoscopic cholecystectomy has not been defined: a systematic review. ANZ J Surg 91:E554–E560. https://doi.org/10.1111/ans.17021
Kim HY, Choi JB, Min SK et al (2021) A randomized clinical trial on the effect of a lidocaine patch on shoulder pain relief in laparoscopic cholecystectomy. Sci Rep 11:1052. https://doi.org/10.1038/s41598-020-80289-y
Bajracharya JL, Subedi A, Pokharel K, Bhattarai B (2019) The effect of intraoperative lidocaine versus esmolol infusion on postoperative analgesia in laparoscopic cholecystectomy: a randomized clinical trial. BMC Anesthesiol 19:198. https://doi.org/10.1186/s12871-019-0874-8
Song X, Sun Y, Zhang X et al (2017) Effect of perioperative intravenous lidocaine infusion on postoperative recovery following laparoscopic cholecystectomy-a randomized controlled trial. Int J Surg 45:8–13. https://doi.org/10.1016/j.ijsu.2017.07.042
Eftekhariyazdi M, Ansari M, Darvishi-Khezri H, Zardosht R (2020) Pharmacological methods of postoperative pain management after laparoscopic cholecystectomy: a review of meta-analyses. Surg Laparosc Endosc Percutan Tech 30:534–541. https://doi.org/10.1097/SLE.0000000000000824
Yang SY, Kang H, Choi GJ et al (2014) Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. J Int Med Res 42:307–319. https://doi.org/10.1177/0300060513505493
Zhao J-B, Li Y-L, Wang Y-M et al (2018) Intravenous lidocaine infusion for pain control after laparoscopic cholecystectomy. Medicine 97:e9771. https://doi.org/10.1097/MD.0000000000009771
Lu J, Wang J-F, Guo C-L et al (2021) Intravenously injected lidocaine or magnesium improves the quality of early recovery after laparoscopic cholecystectomy. Eur J Anaesthesiol 38:S1–S8. https://doi.org/10.1097/EJA.0000000000001348
Li J, Wang G, Xu W et al (2018) Efficacy of intravenous lidocaine on pain relief in patients undergoing laparoscopic cholecystectomy: a meta-analysis from randomized controlled trials. Int J Surg 50:137–145. https://doi.org/10.1016/j.ijsu.2018.01.001
Bazin P, Padley J, Ho M et al (2018) The effect of intravenous lidocaine infusion on bispectral index during major abdominal surgery. J Clin Monit Comput 32:533–539. https://doi.org/10.1007/s10877-017-0035-x
Beaussier M, Delbos A, Maurice-Szamburski A et al (2018) Perioperative use of intravenous lidocaine. Drugs 78:1229–1246. https://doi.org/10.1007/s40265-018-0955-x
Weibel S, Jelting Y, Pace NL et al (2018) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev 6:CD009642. https://doi.org/10.1002/14651858.CD009642.pub3
Ortiz MP, Godoy MC de M, Schlosser RS et al (2016) Effect of endovenous lidocaine on analgesia and serum cytokines: double-blinded and randomized trial. J Clin Anesth 35:70–77.https://doi.org/10.1016/j.jclinane.2016.07.021
Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332. https://doi.org/10.1136/bmj.c332
Ead H (2006) From Aldrete to PADSS: reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 21:259–267. https://doi.org/10.1016/j.jopan.2006.05.006
Chung F, Chan VW, Ong D (1995) A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J Clin Anesth 7:500–506
Allen SM, Madrio ME (2019) Ramsay sedation scale project: small, easy changes for a big effect on patient safety. Crit Care Nurse 39:64–66. https://doi.org/10.4037/ccn2019120
Dong Y, Peng C-YJ (2013) Principled missing data methods for researchers. Springerplus 2:222. https://doi.org/10.1186/2193-1801-2-222
Yang X, Wei X, Mu Y et al (2020) A review of the mechanism of the central analgesic effect of lidocaine. Medicine 99:e19898. https://doi.org/10.1097/MD.0000000000019898
Hermanns H, Hollmann MW, Stevens MF et al (2019) Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth 123:335–349. https://doi.org/10.1016/j.bja.2019.06.014
Kurabe M, Furue H, Kohno T (2016) Intravenous administration of lidocaine directly acts on spinal dorsal horn and produces analgesic effect: an in vivo patch-clamp analysis. Sci Rep 6:26253. https://doi.org/10.1038/srep26253
Eipe N, Gupta S, Penning J (2016) Intravenous lidocaine for acute pain: an evidence-based clinical update. BJA Educ 16:292–298. https://doi.org/10.1093/bjaed/mkw008
Foo I, Macfarlane AJR, Srivastava D et al (2021) The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia 76:238–250. https://doi.org/10.1111/anae.15270
Koppert W, Weigand M, Neumann F et al (2004) Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg 98:1050–1055. https://doi.org/10.1213/01.ANE.0000104582.71710.EE
Marret E, Rolin M, Beaussier M, Bonnet F (2008) Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg 95:1331–1338. https://doi.org/10.1002/bjs.6375
McCarthy GC, Megalla SA, Habib AS (2010) Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 70:1149–1163. https://doi.org/10.2165/10898560-000000000-00000
Cooke C, Kennedy ED, Foo I et al (2019) Meta-analysis of the effect of perioperative intravenous lidocaine on return of gastrointestinal function after colorectal surgery. Tech Coloproctol 23:15–24. https://doi.org/10.1007/s10151-019-1927-1
Sarakatsianou C, Perivoliotis K, Tzovaras G et al (2021) Efficacy of intravenous use of lidocaine in postoperative pain management after laparoscopic colorectal surgery: a meta-analysis and meta-regression of RCTs. In Vivo 35(6):3413–3421. https://doi.org/10.21873/invivo.12641
Sao C-H, Chan-Tiopianco M, Chung K-C et al (2019) Pain after laparoscopic surgery. J Chin Med Assoc 82:819–826. https://doi.org/10.1097/JCMA.0000000000000190
MacFater WS, **a W, Barazanchi AWH et al (2022) Intravenous local anesthetic compared with intraperitoneal local anesthetic in laparoscopic colectomy: a double-blind randomized controlled trial. Ann Surg 275:E30–E36. https://doi.org/10.1097/SLA.0000000000004758
Lauwick S, Do JK, Michelagnoli G et al (2008) Intraoperative infusion of lidocaine reduces postoperative fentanyl requirements in patients undergoing laparoscopic cholecystectomy. Can J Anaesth 55:754–760. https://doi.org/10.1007/BF03016348
Loizides S, Gurusamy KS, Nagendran M et al (2014) Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev 12(3):CD007049. https://doi.org/10.1002/14651858.CD007049.pub2
Abet E, Orion F, Denimal F et al (2017) Interest of using ropivacaine for outpatient laparoscopic cholecystectomy: prospective randomized trial. World J Surg 41:687–692. https://doi.org/10.1007/S00268-016-3797-2
Beaussier M, Parc Y, Guechot J et al (2018) Ropivacaine preperitoneal wound infusion for pain relief and prevention of incisional hyperalgesia after laparoscopic colorectal surgery: a randomized, triple-arm, double-blind controlled evaluation vs intravenous lidocaine infusion, the CATCH study. Colorectal Dis 20:509–519. https://doi.org/10.1111/CODI.14021
Sridhar P, Sistla SC, Ali SM et al (2015) Effect of intravenous lignocaine on perioperative stress response and post-surgical ileus in elective open abdominal surgeries: a double-blind randomized controlled trial. ANZ J Surg 85:425–429. https://doi.org/10.1111/ans.12783
Chia PA, Cannesson M, Bui CCM (2020) Opioid free anesthesia: feasible? Curr Opin Anaesthesiol 33:512–517. https://doi.org/10.1097/ACO.0000000000000878
Gabriel RA, Swisher MW, Sztain JF et al (2019) State of the art opioid-sparing strategies for post-operative pain in adult surgical patients. Expert Opin Pharmacother 20:949–961. https://doi.org/10.1080/14656566.2019.1583743
Hashemian AM, ZamaniMoghadamDoloo H, Saadatfar M et al (2018) Effects of intravenous administration of fentanyl and lidocaine on hemodynamic responses following endotracheal intubation. Am J Emerg Med 36:197–201. https://doi.org/10.1016/j.ajem.2017.07.069
Pustetto M, Goldsztejn N, Touihri K et al (2020) Intravenous lidocaine to prevent endothelial dysfunction after major abdominal surgery: a randomized controlled pilot trial. BMC Anesthesiol 20:155. https://doi.org/10.1186/s12871-020-01075-x
Murthy TKK, Kumar PVV (2018) Effect of perioperative intravenous lignocaine infusion on haemodynamic responses and postoperative analgesia in laparoscopic cholecystectomy surgeries. Anesth Pain Med 24;8(2):e63490. https://doi.org/10.5812/aapm.63490
Gregory A, Stapelfeldt WH, Khanna AK et al (2021) Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg 132:1654–1665. https://doi.org/10.1213/ANE.0000000000005250
Dogan SD, Ustun FE, Sener EB et al (2016) Effects of lidocaine and esmolol infusions on hemodynamic changes, analgesic requirement, and recovery in laparoscopic cholecystectomy operations. Rev Bras Anestesiol 66:145–150. https://doi.org/10.1016/j.bjan.2016.01.004
Yang SS, Wang NN, Postonogova T et al (2020) Intravenous lidocaine to prevent postoperative airway complications in adults: a systematic review and meta-analysis. Br J Anaesth 124:314–323. https://doi.org/10.1016/J.BJA.2019.11.033
Ozcan ATD, Altin CB, Erdogan S et al (2018) The effects of desflurane and sevoflurane on nesfatin-1 levels in laparoscopic cholecystectomy: a randomized controlled trial. BMC Anesthesiol 18(1):23. https://doi.org/10.1186/S12871-018-0484-X
Gangakhedkar G, Monteiro J (2019) A prospective randomized double-blind study to compare the early recovery profiles of desflurane and sevoflurane in patients undergoing laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol 35:53–57. https://doi.org/10.4103/JOACP.JOACP_375_17
Greenwood E, Nimmo S, Paterson H et al (2019) Intravenous lidocaine infusion as a component of multimodal analgesia for colorectal surgery-measurement of plasma levels. Perioper Med (Lond) 8:1. https://doi.org/10.1186/s13741-019-0112-4
Weibel S, Jokinen J, Pace NL et al (2016) Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth 116:770–783. https://doi.org/10.1093/bja/aew101
Author information
Authors and Affiliations
Contributions
Conception and design of the study: Sarakatsianou. Acquisition of data: Georgopoulou, Tsiaka. Analysis and interpretation of data: Perivoliotis. Drafting the article: Perivoliotis, Sarakatsianou. Critical revision: Baloyiannis, Tzovaras.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Hospital of Larissa (UHL 20667, 13/10/17–05-2018).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarakatsianou, C., Perivoliotis, K., Baloyiannis, I. et al. Efficacy of intraoperative intravenous lidocaine infusion on postoperative opioid consumption after laparoscopic cholecystectomy: a randomized controlled trial. Langenbecks Arch Surg 408, 197 (2023). https://doi.org/10.1007/s00423-023-02937-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02937-x