Abstract
Purpose
Textbook outcome (TO) is a composite measure of outcome and provides superior assessment of quality of care after surgery. TO after major living donor hepatectomy (MLDH) has not been assessed. The objective of this study was to determine the rate of TO and its associated factors, after MLDH.
Methods
This was a single center retrospective review of living liver donors who underwent MLDH between 2012 and 2021 (n = 1022). The rate of TO and its associated factors was determined.
Results
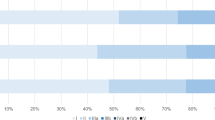
Among 1022 living donors (of whom 693 [67.8%] were males, median age 26 [range, 18–54] years), TO was achieved in 714 (69.9%) with no donor mortality. Majority of donors met the cutoffs for individual outcome measures: 908 (88.8%) for no major complications, 904 (88.5%) for ICU stay ≤ 2 days, 900 (88.1%) for hospital stay ≤ 10 days, 990 (96.9%) for no perioperative blood transfusion, 1004 (98.2%) for no 30-day re-admission, and 1014 (99.2%) for no post-hepatectomy liver failure. Early donation era (before streamlining of donor operative pathways) was associated with failure to achieve TO [OR 1.4, CI 1.1–1.9, P = 0.006]. TO was achieved in 506/755 (67%) donors in the early donation era versus 208/267 (77.9%) in the later period (P = 0.001).
Conclusion
Despite zero mortality and low complication rate, TO was achieved in approximately 70% donors. TO was modifiable and improved with changes in donor operative pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-operative outcomes are an important determinant of healthcare quality. Traditionally, individual measures like morbidity, mortality, hospital stay, and re-admission have been used to assess hospital performance [1]. These individual measures might not capture the multidimensional recovery process in surgical patients. Hospitals might perform well in some while do poorly in other individual metrics. As a result, surgical procedures with low morbidity and mortality might be considered absolutely safe, despite considerable rates of patient dissatisfaction [2].
It has been suggested that composite measures of quality might be superior to individual outcome measures [3, 4]. A desirable outcome is achieved when all these parameters are met in an individual patient. This “all or none approach” to patient care substantially raises the bar, and forms the basis for the newly proposed term text book outcomes (TO) in surgery [5]. The term was first described in 2013 by a group of colorectal surgeons in Netherlands [6]. Recent reports suggest that TO is achieved in 25.5 to 62.3% patients undergoing liver resections. Various patient-, procedural-, and tumor-related factors contribute towards achieving TO in these patients [1] [7,8,9].
In living liver donation (LLD), complex liver surgery is performed with a very low anticipated morbidity and negligible mortality. LLD is based upon the principle of double equipoise which states that the benefit to the recipient must be balanced against the risk to the donor [10, 11]. Worldwide living donor liver transplant (LDLT) programs use stringent criteria for donor selection. This mitigates many patient and procedural factors that might negatively impact outcomes after LLD. For example, donors are young (18–55 years) and healthy, have normal liver function, and retain a minimum acceptable future liver remnant (≥ 30%) to be eligible for donation [12]. Nevertheless, serious morbidity and mortality are reported in 15–20% and 0.5% donors undergoing major hepatectomy respectively [13, 14]. LLD is a major surgical intervention with the sole purpose of saving a loved one’s life. With existing barriers to LLD and ongoing organ shortage, it is important to identify gaps that might negatively impact donor’s experience during the donation process. To this end, assessment of TO can improve quality of care in liver donors, thereby promoting the spirit of donation in other members of the community.
So far, TO have not been reported after major living donor hepatectomy (MLDH). The objective of the current study was to assess TO after MLDH and identify factors associated with TO.
Materials and methods
Study population and data collection
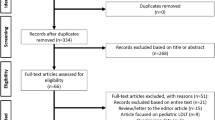
Between April 2012 and July 2021, 1037 living donor hepatectomies (LDH) were performed at our center. These included right hepatectomy (n = 971), left hepatectomy (n = 51), and left lateral hepatectomy (n = 15). Donors were healthy, 18–55 years of age, blood group compatible, and related to the recipient (legal or blood relation).The stepwise donor workup has been discussed elsewhere [15]. The decision to proceed with donation was made by the hospital ethics committee and human organ transplant authority. A donor was considered to have undergone MLDH when the procured graft included ≥ 3 Couinaud segments i.e. right (segments 5, 6, 7, 8) or left (segments 2, 3, 4) hepatectomy [16]. For this study, we reviewed our donor database and all consecutive donors who underwent MLDH (n = 1022) were included.
Textbook outcome
The TO for this study was based on six outcome variables: ICU stay (≤ 2 days), hospital stay (HS) (≤ 10 days), no 30-day major complications, no post-hepatectomy liver failure (PHLF), no perioperative blood transfusion, and no 30-day re-admission. Since there was no post-operative mortality in our cohort, we did not include this as an outcome measure. We used Clavien-Dindo classification for complications and grade 3 and above complications were considered as major complications [17]. Major complications, re-admissions, blood transfusions, and HS have been used previously to determine TO [1, 2] [7, 8]. We included PHLF as an additional outcome measure since it is important for donor wellbeing to have predictable and re-assuring liver functions in the early post-donation period. We used 50–50 criteria to define PHLF since it was simple, liver specific, and easier to calculate when working on a retrospective cohort [18]. We used ICU stay ≤ 2 days and hospital stay ≤ 10 days to define TO as per our institutional policy. With an uneventful postoperative course, donors who underwent a MLDH were shifted from ICU in ≤ 2 days and were discharged in ≤ 10 days of hospital admission. More specifically, once a donor was vitally stable, on room air, mobilizing out of bed, with re-assuring laboratory investigations, a discharge from ICU was considered. Discharge from the hospital was considered once the donor was on regular diet, pain free, had normal or down trending liver functions, and no signs of active infection or any other complications.
We included ICU stay as an additional measure besides hospital stay since prolonged ICU stay is independently associated with poor outcomes after surgery [19, 20]. When all six desired outcomes were met, the donor was considered to have achieved a TO.
Statistical analysis
Categorical variables were presented as frequencies. Based on normal distribution, continuous variables were presented as median and interquartile ranges (IQR) or mean and standard deviation (SD). Demographics (age, sex, BMI) and liver related variables (liver attenuation index (LAI), FLR, hepatitis B core antibody positivity, and graft type) were compared between donors who achieved a TO outcome (TO +) or did not (TO −). The LAI was calculated as the hepatic-to-splenic attenuation ratio, which is obtained by dividing the value of hepatic attenuation by the value of splenic attenuation on noncontract CT scan. LAI is reduced when there is significant liver steatosis. When LAI is 0 or less, it represents significant steatosis and donors with LAI < 1 are not accepted for donation. From January 2019 onwards, certain modifications were introduced in the donor operative pathway (Fig. 1). In order to assess the impact of these modifications, TO was compared in the early (April 2012–December 2018) and late donation era (January 2019-June 2021) of LLD. For categorical variables, Pearson X2 test and Fischer test were used while for continuous variables, t test or Mann–Whitney U test was used. Factors like FLR, graft type, variant portal and biliary anatomy, and MHV inclusion with the graft have been associated with donor outcomes [12] [21,22,23,24,25, a Precut cholangiogram (after liver transection) with bulldog clamp applied near the confluence (yellow arrow) to identify point of division of RHD. b Completion cholangiogram with RHD stump (yellow arrow) after graft procurement to prevent biliary complications in the donor. c A modified right lobe graft with MHV exposure, segment 5 (V5) and segment 8 (V8) veins will be ligated and used for reconstruction
A significant difference was observed between the two eras for ICU and hospital stay, abdominal collections, and wound infections (Table 3). The rates of biliary complications (2.1% to 1.5%), pleural effusions (4.5% to 3.7%), and blood transfusions (3.4% to 1.9%) also reduced in the later era, but the difference was not significant due to smaller number of these events. Contrary to this, there was an increase in re-admission rate in the later era. This can be attributed to the impact of COVID-19. Access to hospitals, healthcare professionals, and transportation was difficult during this time [29]. As a result, we kept a lower threshold for re-admission after LLD. Donors who presented with fever, any respiratory symptoms, or other signs of infection were more likely to be re-admitted when compared with pre COVID era.
Despite zero mortality, we noted that certain deviations from the normal postoperative course including prolonged ICU stay, hospital stay, interventions for biliary complications, and blood transfusions were associated with increased anxiety among donors and their respective families. Considering that LDLT is the only mode of donation in certain regions of the world, it is important that living donors play their role in promoting donation in the community. For this to happen, there is a need to reduce deviation from the ideal postoperative course in healthy voluntary donors. We believe that LDLT centers should strive to achieve a TO in all donors.
There are certain limitations of the current study. The study reports TO from an exclusively LDLT center and the results should be judiciously applied to centers with simultaneous deceased and living donor liver transplant activity. Although streamlining of donor operation significantly improved outcomes, it is not clear which single factor out of all modifications was most crucial. During the later period, there was more rigorous implementation of certain steps pertaining to operative pathway; however, these were not completely new and were partially practiced before streamlining as well. For example, while factors such as designated donor surgeon, 2 surgeon graft procurement, precut cholangiogram, and MHV exposure were only introduced in the later period, modified right lobe grafts were used previously although MHV exposure was not necessary. It is also difficult to ascertain how the increasing experience of the surgical and non-surgical teams contributed to TO. In addition, the cutoff on ICU and hospital stay is center-specific and cannot be generalized. In fact, some donors might be discharged as early as POD 4 after donation but are kept in hospital in view of increased donor safety. As such, the ideal cutoffs are difficult to establish in LDLT. Moreover, it is also important to assess the impact of TO in donors on recipients in future research. This single center analysis merits validation in multicenter studies, yet enables in depth assessment of changing practices and how they impact outcomes in an LDLT program.
Conclusion
Despite achieving individual outcome measures in majority of donors, the overall rate of TO is only acceptable. TO is a simple composite measure of patient care that allows identification of gaps in delivered quality of surgical care. It can be used as a shared decision making tool enabling clinicians and potential donors to have realistic expectations and identify ways to improve the overall surgical experience. More importantly, TO is modifiable, and larger multicenter studies are needed to identify factors that increase rate of TO after LLD.