Abstract
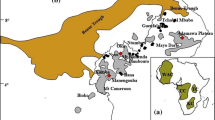
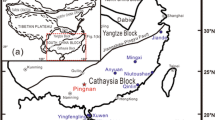
We have investigated the partitioning of Fe3+ between orthopyroxene (Opx) and garnet (Grt) in well-equilibrated mantle xenoliths using Mössbauer spectroscopy. The samples cover a wide range of P–T conditions (2.1–6.6 GPa, 690–1,412 °C) and geothermal gradients, and are thus representative for Earth’s upper mantle in both on-craton and off-craton continental settings. Garnet has Fe3+/Fetot ratios of 0.03–0.13 and Fe2O3 contents of 0.24–1.00 wt%. Orthopyroxene has, on average, lower Fe3+/Fetot ratios (0.01–0.09) and Fe2O3 contents (0.05–0.63 wt%). In low-pressure, high-temperature samples, however, Opx is systematically richer in Fe2O3 than the coexisting Grt. The Fe3+ Opx/Grt partition coefficient \(\left( {D_{{{\text{Fe}}^{ 3+ } }}^{\text{Opx/Grt}} } \right)\) shows no obvious relationship with temperature, but increases with decreasing pressure and with increasing NaOpx. The observed Opx/Grt Fe3+ systematics imply that the Opx–Grt Fe–Mg exchange thermometer is not robust against redox changes if total Fe is treated as Fe2+. An approximate evaluation of errors on T estimates due to redox effects predicts negligible deviations for strongly reduced conditions (<65 °C), but potentially large deviations (> to ≫100 °C) for strongly oxidized conditions, especially at very high pressure and when both P and T are calculated by iteration.









Similar content being viewed by others
References
Amthauer G, Annersten H, Hafner SS (1976) The Mössbauer spectrum of 57Fe in silicate garnets. Z Kristallogr 143:14–55
Annersten H, Olesch M, Seifert FA (1978) Ferric iron in orthopyroxene: a Mössbauer spectroscopic study. Lithos 11:301–310
Boyd FR, Mertzman SA (1987) Composition and structure of the Kaapvaal lithosphere, southern Africa. In: Mysen BO (ed) Magmatic processes: physiochemical principles, vol 1. Geochemical Society Special Publication, pp 13–24
Brey GP, Köhler T (1990) Geothermobarometry in four-phase lherzolites II. New thermobarometers, and practical assessment of existing thermobarometers. J Petrol 31:1353–1378
Canil D, O’Neill HSC (1996) Distribution of ferric iron in some upper-mantle assemblages. J Petrol 37:609–635
Caro G (2000) Petrography of the Kennedy Lake orangeite and its mantle xenoliths. Unpublished MSc thesis, University of British Columbia, 100 p
Carswell DA (1991) The garnet-orthopyroxene Al barometer: problematic application to natural garnet lherzolite assemblages. Mineral Mag 55:19–31
Cox KG, Smith MR, Beswetherick S (1987) Textural studies of garnet lherzolites: evidence of exsolution origin from high-temperature harzburgites. In: Nixon PH (ed) Mantle xenoliths. Wiley, Chichester, pp 537–550
Creighton S, Stachel T, Matveev S, Höfer HE, McCammon C, Luth RW (2009) Oxidation of the Kaapvaal lithospheric mantle driven by metasomatism. Contrib Mineral Petrol 157:491–504
Creighton S, Stachel T, Eichenberg D, Luth RW (2010) Oxidation state of the lithospheric mantle beneath Diavik diamond mine, central Slave craton, NWT, Canada. Contrib Mineral Petrol 159:645–657
Dollase WA (1975) Statistical limitations of Mössbauer spectral fitting. Am Mineral 60:257–264
Domeneghetti MC, Molin GM, Tazzoli V (1995) A crystal-chemical model for PBCA orthopyroxene. Am Mineral 80:253–267
Doucet LS, Ionov DA, Golovin AV (2013) The origin of coarse garnet peridotites in cratonic lithosphere: new data on xenoliths from the Udachnaya kimberlite, central Siberia. Contrib Mineral Petrol 165:1225–1242
Doucet LS, Peslier AH, Ionov DA, Brandon AD, Golovin AV, Goncharov AG, Ashchepkov IV (2014) High water contents in the Siberian cratonic mantle linked to metasomatism: an FTIR study of Udachnaya peridotite xenoliths. Geochim Cosmochim Acta 137:159–187
Frost DJ, McCammon CA (2008) The redox state of Earth’s mantle. Annu Rev Earth Planet Sci 36:389–420
Goncharov AG, Ionov DA (2012) Redox state of deep off-craton lithospheric mantle: new data from garnet and spinel peridotites from Vitim, southern Siberia. Contrib Mineral Petrol 164:731–745
Goncharov AG, Ionov DA, Doucet LS, Pokhilenko LN (2012) Thermal state, oxygen fugacity and C–O–H fluid speciation in cratonic lithospheric mantle: new data on peridotite xenoliths from the Udachnaya kimberlite, Siberia. Earth Planet Sci Lett 357–358:99–110
Grégoire M, Bell DR, le Roux AP (2003) Garnet lherzolites from the Kaapvaal craton (South Africa): trace element evidence for a metasomatic history. J Petrol 44:629–657
Gudmundsson G, Wood BJ (1995) Experimental tests of garnet peridotite oxygen barometry. Contrib Mineral Petrol 119:56–67
Harley SL (1984) An experimental study of the partitioning of Fe and Mg between garnet and orthopyroxene. Contrib Mineral Petrol 86:359–373
Hops JJ, Gurney JJ, Harte B, Winterburn P (1989) Megacrysts and high temperature nodules from the Jagersfontein kimberlite pipe. In: Ross J (ed) Kimberlites and related rocks, vol 2, their mantle/crust setting, diamonds and diamond exploration. Geological Society of Australia Special Publication, vol 14, pp 759–770
Ionov DA (2002) Mantle structure and rifting processes in the Baikal–Mongolia region: geophysical data and evidence from xenoliths in volcanic rocks. Tectonophysics 351:41–60
Ionov DA, Griffin WL, O’Reilly SY (1999) Off-cratonic garnet and spinel peridotite xenoliths from Dsun-Bussular, SE Mongolia. In: Gurney JJ, Gurney JL, Pascoe MD, Richardson SH (eds) Proceedings of 7th International Kimberlite Conference, vol 1. Red Roof Design Cape Town, pp 383–390
Ionov DA, Doucet LS, Ashchepkov IV (2010) Composition of the lithospheric mantle in the Siberian craton: new constraints from fresh peridotites in the Udachnaya-East kimberlite. J Petrol 51:2177–2210
Kopylova MG, Caro G (2004) Mantle xenoliths from the southeastern Slave craton: evidence for chemical zonation in a thick, cold lithosphere. J Petrol 45:1045–1067
Kopylova MG, Russel JK, Cookenboo H (1999a) Petrology of peridotite and pyroxenite xenoliths from the Jericho kimberlite: implications for the thermal state of the mantle beneath the Slave craton, Northern Canada. J Petrol 40:79–104
Kopylova MG, Russel JK, Cookenboo H (1999b) Map** the lithosphere below the north central Slave craton. In: Gurney JJ, Gurney JL, Pascoe MD, Richardson SH (eds) Proceedings of 7th International Kimberlite Conference, vol 1. Red Roof Design Cape Town, pp 468–479
Lazarov M, Woodland AB, Brey GP (2009) Thermal state and redox conditions of the Kaapvaal mantle: a study of xenoliths from the Finsch mine, South Africa. Lithos 112S:913–923
Luth RW, Canil D (1993) Ferric iron in mantle-derived pyroxenes and a new oxybarometer for the mantle. Contrib Mineral Petrol 113:236–248
Luth RW, Virgo D, Boyd FR, Wood BJ (1990) Ferric iron in mantle-derived garnets. Implications for thermobarometry and for the oxidation state of the mantle. Contrib Mineral Petrol 104:56–72
Malaspina N, Langenhorst F, Fumagalli P, Tumiati S, Poli S (2012) Fe3+ distribution between garnet and pyroxenes in mantle wedge carbonate-bearing garnet peridotites (Sulu, China) and implications for their oxidation state. Lithos 146–147:11–17
Matjuschkin V, Brey GP, Höfer HE, Woodland AB (2014) The influence of Fe3+ on garnet–orthopyroxene and garnet–olivine geothermometers. Contrib Mineral Petrol 167:1–10
McCammon C, Kopylova MG (2004) A redox profile of the Slave mantle and oxygen fugacity control in the cratonic mantle. Contrib Mineral Petrol 148:55–68
McCammon CA, Tennant WC, Miletich RM (2000) A new method for single crystal measurements: application to studies of mineral inclusions in diamonds. Hyper Inter 126:241–245
McCammon CA, Griffin WL, Shee SR, O’Neill HSC (2001) Oxidation during metasomatism in ultramafic xenoliths from the Wesselton kimberlite, South Africa: implications for the survival of diamond. Contrib Mineral Petrol 141:287–296
Mofokeng SW (1998) A comparison of the nickel and the conventional geothermometers with respect to the Jagersfontein and the Matsoku kimberlite peridotite xenoliths. M Sc Thesis, University of Cape Town, 124 p
Nickel KG (1989) Garnet-pyroxene equilibria in the system SMACCR (SiO2–MgO–Al2O3–CaO–Cr2O3): the Cr-geobarometer. In Ross J (ed) Kimberlites and related rocks, vol 2, their mantle/crust setting, diamonds and diamond exploration. Proceedings of 4th International Kimberlite Conference. Geological Society of Australia Special Publication, vol 14, pp 901–912
Nickel KG, Green DH (1985) Empirical geothermobarometry for garnet peridotites and implications for the nature of the lithosphere, kimberlites and diamonds. Earth Planet Sci Lett 73:158–170
Nimis P, Grütter H (2010) Internally consistent geothermometers for garnet peridotites and pyroxenites. Contrib Mineral Petrol 159:411–427
Nimis P, Taylor WR (2000) Single-clinopyroxene thermobarometry for garnet peridotites. Part I. Calibration and testing of a Cr-in-Cpx barometer and an enstatite-in-Cpx thermometer. Contrib Mineral Petrol 139:541–554
O’Neill HSC, Wall VJ (1987) The olivine–orthopyroxene–spinel oxygen geobarometer, the nickel precipitation curve, and the oxygen fugacity of the earth’s upper mantle. J Petrol 28:1169–1191
Pearson DG, Boyd FR, Haggerty SE, Pasteris JD, Field SW, Nixon PH, Pokhilenko NP (1994) The characterisation and origin of graphite in cratonic lithospheric mantle: a petrological carbon isotope and Raman spectroscopic study. Contrib Mineral Petrol 115:449–466
Pollack HN, Chapman DS (1977) On the regional variations of heat flow, geotherms and lithospheric thickness. Tectonophysics 38:279–296
Purwin H, Lauterbach S, Brey GP, Woodland AB, Kleebe H-J (2013) An experimental study of the Fe oxidation states in garnet and clinopyroxene as a function of temperature in the system CaO–FeO–Fe2O3–MgO–Al2O3–SiO2: implications for garnet–clinopyroxene geothermometry. Contrib Mineral Petrol 165:623–639
Saltzer RL, Chatterjee N, Grove TL (2001) The spatial distribution of garnets and pyroxenes in mantle peridotites: pressure–temperature history of peridotites from the Kaapvaal craton. J Petrol 42:2215–2229
Schmidberger SS, Francis D (1999) Nature of mantle roots beneath the North American craton: mantle xenolith evidence from Somerset Island kimberlites. Lithos 48:195–216
Shirey SB, Cartigny P, Frost DJ, Keshav S, Nestola F, Nimis P, Pearson DG, Sobolev NV, Walter MJ (2013) Diamonds and the geology of mantle carbon. Rev Mineral Geochem 75:355–421
Smith D (1999) Temperatures and pressures of mineral equilibration in peridotite xenoliths: review, discussion, and implications. In: Fei Y, Bertka CM, Mysen BO (eds) Mantle petrology: field observations and high pressure experimentation: a tribute to Francis R. (Joe) Boyd, vol 6. Geochemical Society Special Publication, pp 171–188
Stagno V, Ojwang DO, McCammon CA, Frost DJ (2013) The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 493:84–88
Taylor WR (1998) An experimental test of some geothermometer and geobarometer formulations for upper mantle peridotites with application to the thermobarometry of fertile lherzolite and garnet websterite. Neues Jb Miner Abh 172:381–408
Woodland AB (2009) Ferric iron contents of clinopyroxene from cratonic mantle and partitioning behaviour with garnet. Lithos 112S:1143–1149
Woodland AB, Koch M (2003) Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal craton, Southern Africa. Earth Planet Sci Lett 214:295–310
Woodland AB, Peltonen P (1999) Ferric iron contents of garnet and clinopyroxene and estimated oxygen fugacities of peridotite xenoliths from the Eastern Finland Kimberlite Province. In JJ Gurney, JL Gurney, MD Pascoe, SH Richardson (eds) Proceedings of 7th International Kimberlite Conference, vol 2. Red Roof Design Cape Town South Africa, pp 904–911
Woodland AB, Ross CR (1994) A crystallographic and Mössbauer spectroscopy study of Fe3Al2Si3O12–Fe 2+3 Fe 3+2 Si3O12 (almandine-skiagite) and Ca3Fe 3+2 Si3O12–Fe 2+3 Fe 3+2 Si3O12 (andradite-skiagite) garnet solid solutions. Phys Chem Minerals 21:117–132
Yaxley GM, Berry AJ, Kamenetsky VS, Woodland AB, Golovin AV (2012) An oxygen fugacity profile through the Siberian Craton—Fe K-edge XANES determinations of Fe3+/∑Fe in garnets in peridotite xenoliths from the Udachnaya East kimberlite. Lithos 140–141:142–151
Acknowledgments
We are grateful to Dante Canil for providing access to his original data set. Sula Milani is thanked for her help in retrieving the old Mössbauer files. Formal reviews by Bob Luth and two anonymous referees helped us to improve the paper. PN acknowledges financial support by MIUR ex60%. DAI thanks Igor Ashchepkov for providing some of the xenoliths used in this study and acknowledges financial support from the French CNRS, including PNP-INSU and PICS grants, and from the Australian Research Council including Research fellowship and Grants in 1994–1998.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Ballhaus.
Appendix: Estimation of maximum bias on Opx–Grt temperature estimates due to changing redox conditions
Appendix: Estimation of maximum bias on Opx–Grt temperature estimates due to changing redox conditions
Figure 8 shows a compilation of existing fO2 data for mantle xenoliths worldwide, recalculated using input P–T values obtained with the thermobarometer combinations recommended by Nimis and Grütter (2010). This choice significantly reduced the scatter of points (especially for Diavik) compared to earlier published versions of this plot (e.g., Stagno et al. 2013). Correction of Canil and O’Neill’s (1996) Mössbauer data for different recoil-free fractions in Grt (Table 4) produced a slight decrease in calculated fO2 of about 0.6 Δlog units. The plot shows the well-known overall decrease in FMQ-normalized oxygen fugacity with increasing mantle depth and a range for fO2 at each depth.
From this compilation, we selected five xenoliths coming from different depths and recording “average” redox conditions for their particular depths of provenance (Table 6; Fig. 8). We calculated the Opx–Grt temperatures for these xenoliths with the thermometer version of Nimis and Grütter (2010) (hereafter TNG10) at P given by the thermobarometers combination recommended by the same authors, using total Fe. The TNG10 thermometer was calibrated against a large set of mantle xenoliths from localities worldwide and should therefore be robust when applied to mantle rocks characterized by “average” redox conditions. All selected xenoliths showed very good agreement (ΔT < 60 °C) between thermometric estimates using the internally consistent thermometers recommended by Nimis and Grütter (2010). This indicates good equilibrium and also confirms that redox conditions in the xenoliths were indeed “average” and compatible with the TNG10 thermometer calibration (cf Nimis and Grütter 2010). Therefore, the calculated P–T conditions should be reliable.
We then allowed fO2 for each of the selected xenoliths to vary to the maximum and minimum values expected for the mantle at the corresponding depths, as indicated by our compilation in Fig. 8. We estimated the Fe3+/Fetot ratios in the garnets at these maximum and minimum redox conditions by reversing the oxybarometer of Stagno et al. (2013), and those in the coexisting orthopyroxenes by using the Fe3+ partitioning systematics obtained in our work (cf Eq. 6). The mineral compositions were modified using the new Fe3+/Fetot ratios while kee** \(K_{{D_{{{\text{Fe}}^{2 + } - {\text{Mg}}}} }}^{{{\text{Grt}} - {\text{Opx}}}}\) unvaried––the latter depends essentially on T, therefore kee** it fixed corresponds to kee** T fixed. An increase (or decrease) in the Fe3+/Fetot ratio thus determined a net increase (or decrease) in the total Fe content (actually Fe3+), which was compensated by varying the Al3+ + Cr3+ contents by the same magnitude at constant Al/Cr ratio. Since the solid solution model for garnet which is used in the oxybarometer of Stagno et al. (2013) is sensitive to the Al and Cr contents, the Fe3+/Fetot ratios had to be readjusted by iteration, although the effect of this correction was found to be minimal.
We then recalculated the TNG10 temperatures using the modified total Fe contents in both orthopyroxenes and garnets, either kee** P fixed or recalculating both P and T iteratively. The P–T estimates obtained for the selected xenoliths using the original mineral compositions and the compositions modified for their respective maximum and minimum redox conditions are reported in Table 6. We emphasize that the aim of this exercise was to assess “relative” variations on final P–T estimates and that possible small interlab discrepancies in Fe3+/Fetot ratios for Grt extracted from the literature and from this work do not significantly alter our results.
Rights and permissions
About this article
Cite this article
Nimis, P., Goncharov, A., Ionov, D.A. et al. Fe3+ partitioning systematics between orthopyroxene and garnet in mantle peridotite xenoliths and implications for thermobarometry of oxidized and reduced mantle rocks. Contrib Mineral Petrol 169, 6 (2015). https://doi.org/10.1007/s00410-014-1101-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-014-1101-8