Abstract
Progressive supranuclear palsy (PSP) is a 4R-tauopathy predominated by subcortical pathology in neurons, astrocytes, and oligodendroglia associated with various clinical phenotypes. In the present international study, we addressed the question of whether or not sequential distribution patterns can be recognized for PSP pathology. We evaluated heat maps and distribution patterns of neuronal, astroglial, and oligodendroglial tau pathologies and their combinations in different clinical subtypes of PSP in postmortem brains. We used conditional probability and logistic regression to model the sequential distribution of tau pathologies across different brain regions. Tau pathology uniformly predominates in the neurons of the pallido-nigro-luysian axis in different clinical subtypes. However, clinical subtypes are distinguished not only by total tau load but rather cell-type (neuronal versus glial) specific vulnerability patterns of brain regions suggesting distinct dynamics or circuit-specific segregation of propagation of tau pathologies. For Richardson syndrome (n = 81) we recognize six sequential steps of involvement of brain regions by the combination of cellular tau pathologies. This is translated to six stages for the practical neuropathological diagnosis by the evaluation of the subthalamic nucleus, globus pallidus, striatum, cerebellum with dentate nucleus, and frontal and occipital cortices. This system can be applied to further clinical subtypes by emphasizing whether they show caudal (cerebellum/dentate nucleus) or rostral (cortical) predominant, or both types of pattern. Defining cell-specific stages of tau pathology helps to identify preclinical or early-stage cases for the better understanding of early pathogenic events, has implications for understanding the clinical subtype-specific dynamics of disease-propagation, and informs tau-neuroimaging on distribution patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive supranuclear palsy (PSP) is a four-repeat (4R) tauopathy that belongs to the group of frontotemporal lobar degeneration (FTLD-tau) disorders [34]. The neuropathological diagnosis of PSP is based on the presence of neurofibrillary tangles and threads in subcortical nuclei together with the presence of tufted astrocytes [5, 13]. In addition, oligodendroglial coiled bodies and diffuse cytoplasmic immunoreactivity in neurons can be observed as well [9, 18]. Following the proposal of typical, atypical, and combined cases of PSP by Lantos [26], Williams et al. provided evidence for biochemical and tau pathology load differences between PSP with Parkinsonism (PSP-P) and the classical clinical phenotype Richardson syndrome (PSP-RS) [47, 48]. Soon thereafter, further clinical phenotypes with PSP-type tau pathology were described [6]. In 2017, the Movement Disorders Society suggested clinical diagnostic criteria to recognize these distinct clinical subtypes as PSP-RS, PSP-P, PSP with corticobasal syndrome (PSP-CBS), PSP with progressive gait freezing (PSP-PGF), PSP with predominant ocular motor dysfunction (PSP-OM), with predominant postural instability (PSP-PI), with predominant frontal presentation (PSP-F), or with predominant speech and language disorder (PSP-SL) [14].
Neuropathological studies on smaller cohorts suggest differences in the burden of tau pathology between clinical subtypes. Williams et al. noted in 2007 that the mean severity of pathology in all regions of the PSP-RS group (n = 22) was higher than in PSP-P (n = 14) and PSP with pure akinesia with gait freezing (currently called PSP-PGF, n = 6), and the overall tau load was significantly higher in PSP-RS than in PSP-P. Sakae et al. compared PSP-RS (n = 31) cases with PSP-F (n = 15) and found increased tau burden only in the superior frontal gyrus gray matter and inferior temporal gyrus white matter in PSP-F [36]. Tsuboi et al. examined cases (n = 5) presenting with PSP-CBS and concluded that this is most likely due to either concurrent cortical pathology, or to the primary pathology of PSP affecting cortical areas that are primarily and commonly affected by corticobasal degeneration (CBD), another 4R tauopathy [45]. Ling et al. also examined PSP-CBS cases (n = 10) and demonstrated that the overall severity of tau pathology was the same between PSP-CBS and PSP-RS but with a shift of tau burden towards the cortical regions [27]. On the other hand, the rare PSP-PGF variant showed almost no cortical tau pathology, but severe degeneration of the globus pallidus, substantia nigra, and subthalamic nucleus, hence called also pallido-nigro-luysian degeneration [1].
In addition to the recognition of clinical subtypes, a novel concept raises the possibility of propagation of pathological tau in PSP as well as other tauopathies [11] providing a potential therapeutic target [16, 34]. Indeed, sequential distribution patterns have been recognized for tau pathologies such as neurofibrillary degeneration in Alzheimer’s disease (AD) [3], Pick’s disease [15], argyrophilic grain disease [35], or astrocytic tau pathologies [15, Full size image
Next, we compared the load of different cellular tau pathologies in clinical subtypes. Neuronal tau pathology affects mostly brainstem and subcortical nuclei but involvement of the amygdala and hippocampus is also considerable in all subtypes (Fig. 3a). Astroglial tau pathology clearly predominates in cortical areas and striatum and shows differences between clinical subtypes in all cortical areas, thalamus/subthalamus and substantia nigra (Fig. 3b). Furthermore, accumulation of oligodendroglial tau pathology is characteristic in subcortical nuclei and shows variability between subtypes in most of the cortical and subcortical and brainstem regions and cerebellum (Fig. 3c). Mann–Whitney test reveals significant differences between subtypes in several brain regions for each neuronal, astroglial, and oligodendroglial tau pathologies (Table 3). In logistic regression models, the duration of illness did not show any effect on these differences. Adding the presence of AD type pathology (i.e., presence of plaques), AGD, and age showed an effect on the results of differences of neuronal tau accumulation in the occipital and premotor cortex, amygdala and hippocampus but not in the frontal, parietal, and temporal cortices. While comparison of each clinical phenotype showed differences of at least one tau cytopathology in at least one region, major differences were noted in astroglial and oligodendroglial tau accumulation between PSP-RS and PSP-P, PSP-RS and PSP-PI, PSP-F and PSP-P, PSP-F and PSP-PI, PSP-PI and PSP-P, PSP-P and PSP-CBS, PSP-PI and PSP-SL and PSP-PI and PSP-CBS (Table 3). Neuronal tau accumulation was different mostly between PSP-RS and PSP-P, PSP-RS and PSP-PI, PSP-RS and PSP-SL, PSP-PI and PSP-F, PSP-P and PSP-PI and PSP-PI and PSP-CBS (Table 3). Neuronal loss correlated well with total tau load only in subcortical and brainstem regions and not in neocortical areas, likely due to the fact that cortical tau pathology was predominated by astroglial tau.
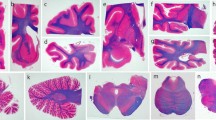
Graphic representation of semiquantitative scores of different cellular tau pathologies (a neuronal, b astroglial, c oligodendroglial) in PSP clinical subtypes. To avoid overcrowding of the graphs, three subtypes are shown in bars and three with lines. Significant (p < 0.05) results of Mann–Whitney test are shown for each comparison in D (blue box indicates neuronal, N, red box for astroglial, A, and green for oligodendroglial, O). OC Occipital cortex, TE temporal cortex, PA parietal cortex, FR frontal cortex, MC motor cortex, AM amygdala, HI hippocampus, ST striatum, TH thalamus and subthalamic nucleus, GP globus pallidus, TG midbrain tegmentum, SN substantia nigra, LC locus coeruleus, PB pontine base, MO medulla oblongata, DE/CB dentate nucleus and cerebellar white matter
The sum of the three different cellular tau pathologies is the highest in the striatum and the thalamus/subthalamic nucleus and the frontal, parietal, and motor cortices (exemplified by PSP-RS, PSP-P, PSP-F, PSP-PI, see online supplemental file, Figs. 2, 3, 4). This is due to the fact that brainstem nuclei accumulate less glial tau pathologies or only one type, such as oligodendroglial in the globus pallidus, pontine base, or cerebellum, and dentate nucleus.
Heatmap showing the development of tau pathology based on conditional probability matrix of total tau pathology scores in pooled cases of different clinical subtypes. The dark red color indicates early and the yellow-white later involvement. Areas colored with gray were not included in the present study
Finally, we evaluated the combined effect of age, duration of illness, presence of AD-related pathology (plaques), Braak NFT stage and presence of AGD pathology on regional cellular tau pathologies. AGD pathology was found in 78 out 194 cases where diagnostically relevant regions were available for examination (40.2%, no difference between clinical phenotypes according to Chi2 test). Presence of AGD and Braak NFT stage showed significantly higher (p = 0.002 and 0.007, respectively) OR values for hippocampal neuronal tau pathology (OR 25.03, 95% CI 3.11–200.8, and 2.14, 95% CI 1.23–3.72, respectively). Neuronal and oligodendroglial tau pathology in the amygdala was significantly associated with the presence of AGD pathology (p = 0.0001, OR 10.16, 95% CI 2.8–35.6 and p = 0.006, OR 5.4 95% CI 1.6–18.6, respectively). Presence of astroglial tau pathology in the amygdala did not associate with presence of AGD (p = 0.4) or other variables in the model. Occipital cytopathologies were not influenced by these variables in this model.
In summary, total tau load is in general less in the cortex in PSP-P (and PSP-PGF in two cases) and more prominent in PSP-SL (Fig. 2). However, tau pathology in PSP shows clinical subtype and cell type-specific differences in its anatomical distribution. As general rules, the following were observed:
-
1.
Subcortical and brainstem nuclei are most vulnerable for neuronal tau accumulation; however, the motor cortex is also involved;
-
2.
Cortical areas and the striatum are characterized by predominance of astroglial tau pathology;
-
3.
Oligodendroglial tau shows the most variability in clinical subtypes; and finally,
-
4.
It is important to note that the amygdala and hippocampus consistently show tau pathology; however, neuronal tau pathology might indicate concomitant primary age-related tauopathy (PART) or AD or AGD.
Conditional probability analysis for pooled PSP cases
We included in sum 165 pooled cases of PSP-RS, PSP-P, PSP-F, PSP-SL, PSP, PI, and PSP-CBS for this analysis. Our aim with this model was to evaluate whether the accumulation of tau pathology in a certain region precedes another, irrespective of the type of cellular tau pathology (online supplemental file, Figs. 5, 6). According to this, high or substantial evidence exists that involvement of subcortical regions precede cortical areas (first towards fronto-parietal cortices and then to temporal and finally occipital cortices) and dentate nucleus/cerebellar white matter (Fig. 4). This is reminiscent of the distribution map presented by Williams et al., in 2007, but the recognition of mild involvement of the occipital cortex found in our study expands beyond the regions shown there [48].
Distribution map of cellular tau pathology semiquantitative scores (0–3) in PSP-RS cases (n = 81). White box indicates score 0, yellow score 1, orange score 2, and red score 3 for semiquantitative scoring. Presence of Lewy-body pathology above Braak stage 2 or TDP-43 proteinopathy is indicated + , absence by −. Black boxes indicate that that anatomical region was not examined. On the right the proposed stages are indicated. Blue outlined boxes highlight the anatomical regions which were considered for the staging. OC Occipital, TE temporal, PA parietal, FR frontal, MC motor cortex, AM amygdala, HI hippocampus, ST striatum, TH/STN thalamus and subthalamic nucleus, GP globus pallidus, TG midbrain tegmentum, SN substantia nigra, LC locus coeruleus, PB pontine base, MO medulla oblongata, DE/Cbll dentate nucleus and cerebellar white matter
Summary of the conditional probability analyses (see online supplemental file). The upper image represents whether the anatomical regions listed in the left show high, substantial, or moderate probability with a p significance value below 0.01 to precede the involvement of the anatomical regions listed on the top. The lower image represents whether the anatomical regions listed in the left show high, substantial, or moderate probability with a p significance value below 0.05 or fair probability (with p < 0.01) to precede the involvement of the anatomical regions listed on the top.: N neuronal, A astroglial, O oligodendroglial, OC Occipital, TE temporal, PA parietal, FR frontal, MC motor cortex, AM amygdala, HI hippocampus, ST striatum, TH thalamus and subthalamic nucleus, GP globus pallidus, TG midbrain tegmentum, SN substantia nigra, LC locus coeruleus, PB pontine base, MO medulla oblongata, DE/CB dentate nucleus and cerebellar white matter
Relation of tau pathological variables in different anatomical regions in Richardson syndrome
To predict early vulnerable cellular populations, we were next interested to discern which anatomical regions are affected when most others are spared any type of tau pathology. This analysis (Fig. 5) showed for PSP-RS (n = 81) that the subcortical nuclei (striatum, globus pallidus, subthalamic nucleus, and thalamus) and selected brainstem nuclei (substantia nigra, locus coeruleus, and medulla oblongata) show tau pathology in all cases. If the amount of tau pathology is low, then it was always neuronal rather than glial tau pathology in these regions. A single case with PSP-RS showed small amounts of neuronal tau pathology in the subthalamic nucleus, striatum, substantia nigra, and globus pallidus together with Lewy body and TDP-43 pathology. This observation supports the notion that these regions are early vulnerable regions for the development of the PSP-RS clinical phenotype. Following the development of a staging system (see below) we added the proposed stages to the cases (Fig. 5).
We provide the detailed table of conditional probabilities for PSP-RS cases together with the frequencies of low (0 and 1) and high (2 and 3) scores for each anatomical region and tau cytopathology in the online supplemental file Fig. 7, and here summarize the combined interpretation. Since the motor cortex was not examined in all cases of RS and the conditional probability results were similar as for the frontal cortex we show only the results of the frontal cortex.
Sequences of PSP related tau pathology based on the conditional probability matrix and stratified for accumulation of neuronal, astroglial, and oligodendroglial tau pathologies. Note that frontal lobe includes frontal and motor cortices. Note that neuronal tau pathology is frequently seen in the hippocampus and locus coeruleus in stage 1; however, eventually this may be related to concomitant Alzheimer’s disease or primary age-related tauopathy (PART) pathogenesis. To indicate that locus coeruleus is frequently affected early, but alone might be associated with other disease conditions such as AD/PART, we used italic letters
Figure 6 demonstrates which cellular tau pathology in which anatomical region precedes one another with various degrees of likelihood. This shows that accumulation of neuronal tau pathology in the substantia nigra, midbrain tegmentum, locus coeruleus, pontine base, medulla oblongata, globus pallidus and subthalamic nucleus, and thalamus precedes any type of tau pathology in neocortical regions. Accumulation of neuronal tau in these regions precedes accumulation of neuronal tau in the dentate nucleus. Neuronal tau pathology accumulates later in the striatum but precedes neuronal tau accumulation in the parietal, temporal, and occipital cortices. The amygdala and hippocampus show accumulation of neuronal pathology earlier than neocortical neuronal tau. Amygdala is preceded by the accumulation of neuronal tau in the subthalamic nucleus and brainstem nuclei and hippocampus is preceded by the substantia nigra and locus coeruleus. In the cortex, accumulation of neuronal tau in the frontal cortex precedes that in the parietal and occipital cortices. Neuronal tau in the hippocampus and amygdala precedes accumulation of tau pathologies in cortical areas except for astroglial tau in the parietal and frontal cortices. However, early hippocampal involvement might be related to PART or AD pathology.
The striatum is different from other subcortical and brainstem nuclei, since here astroglial tau pathology accumulates more and earlier than neuronal tau pathology in the striatum and this occurs in parallel to the neuronal tau accumulation in other subcortical and brainstem nuclei. Astroglial tau pathology in the striatum precedes astroglial tau pathology in neocortical areas and thalamus. Importantly, in neocortical areas, except for the occipital cortex, significant values in conditional probability analyses suggest that astroglial tau pathology precedes the accumulation of neuronal or oligodendroglial tau pathology. Astroglial tau pathology accumulates later in the parietal, temporal, and occipital cortices, brainstem nuclei, hippocampus, and amygdala than in the frontal cortex. Astroglial tau in the occipital lobe and midbrain tegmentum precedes the locus coeruleus, medulla oblongata or dentate nucleus.
Finally, oligodendroglial tau pathology accumulates in the globus pallidus early and prominently and conditional probability analyses reveal a similar pattern as seen for the accumulation of neuronal tau. Oligodendroglial tau pathology in other regions does not seem to be accumulating early in the disease. Involvement of the subthalamic nucleus and thalamus and the striatum precedes neocortical areas and brainstem. Based on conditional probability analyses in the cortex the sequence seems to be frontal and parietal to temporal and occipital lobe.
Binary logistic regression was performed with multiple variables (i.e., age, duration, and presence of AD type pathology) to evaluate the odds ratios that two compared anatomical regions show accumulation of tau pathology simultaneously. This analysis confirms that neocortical areas are affected only if subcortical and brainstem nuclei are also affected (online supplemental file, Fig. 8). Importantly, it also shows that the involvement of the hippocampus and locus coeruleus is independent from the involvement of strategic subcortical and brainstem nuclei. On the other hand, tau pathology in the amygdala accumulates together with neocortical regions.
Proposed staging schema for the neuropathological practice. −/ + Indicates single cell involvement; + indicates mild; + + / + + + indicates moderate/severe involvement. GP globus pallidus, STN subthalamic nucleus, STR striatum, FR frontal, DE/CB dentate nucleus and cerebellar white matter, OC occipital. This can be applied to all clinical subtypes. The evaluator should focus on different cell types in different brain regions: in GP and DE/CB neuronal (N) or oligodendroglial (O); in the STN neuronal; in the STR and FR and OC cortices astroglial (A). The brain schema is a conceptual summary of the tabularized schema in the lower panel; thus the color coding of different brain regions reflect the variability in scores (or-or) required for a stage
To summarize
Tau pathology in PSP-RS begins with neuronal tau accumulation in subcortical and brainstem nuclei along with early oligodendroglial involvement in the globus pallidus and astroglial involvement in the striatum. This is followed by astroglial tau accumulation in cortical areas, which precedes cortical neuronal and oligodendroglial tau accumulation, altogether following a fronto-parietal to temporal to occipital sequence.