Abstract
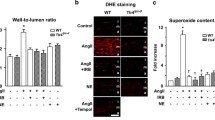
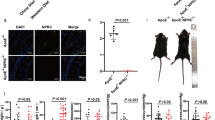
Mice with a global deletion of α1AMPK are characterized by endothelial dysfunction and NADPH oxidase subunit 2 (NOX-2)-mediated vascular oxidative stress. However, the underlying mechanisms are incompletely understood and may involve endothelial NOX-2 upregulation or facilitated vascular infiltration of phagocytic cells. Therefore, the current study was designed to investigate the vascular effects of chronic angiotensin II (AngII) infusion in mice with an endothelial-specific α1AMPK deletion. A mouse strain with endothelial-specific α1AMPK deletion was generated by breeding α1AMPKflox/flox mice with TekCre+ or Cadh5Cre+ mice. Chronic AngII infusion (0.5 mg/kg/day for 7day) caused mild endothelial dysfunction in wild-type mice that was significantly aggravated in endothelial α1AMPK knockout mice. Aortic NOX-2 and CD68 expression were increased, indicating that infiltrating leukocytes may significantly contribute to enhanced vascular oxidative stress. Flow cytometry revealed a higher abundance of aortic CD90.2+ T-cells, CD11b+F4/80+ macrophages and Ly6G−Ly6C+ monocytes. Vascular mRNA expression of monocyte chemoattractant protein 1, CCL5 and vascular cell adhesion molecule 1 was enhanced in AngII-infused mice lacking endothelial α1AMPK, facilitating the recruitment of inflammatory cells to the vessel wall. In addition, AngII-induced upregulation of cytoprotective heme oxygenase 1 (HO-1) was blunted in mice with endothelial α1AMPK deletion, compatible with an impaired antioxidant defense in these animals. In summary, endothelial expressed α1AMPK limits the recruitment of inflammatory cells to the vessel wall and maintains HO-1 mediated antioxidant defense. Both mechanisms reduce vascular oxidative damage and preserve endothelial function during chronic AngII treatment.






Similar content being viewed by others
Abbreviations
- ACh:
-
Acetylcholine
- AMP:
-
Adenosine monophosphate
- AMPK:
-
AMP-activated protein kinase
- AngII:
-
Angiotensin II
- CCR2:
-
C–C chemokine receptor type 2
- CCL5:
-
CC-chemokine ligand 5
- DHE:
-
Dihydroethidium
- eNOS:
-
Endothelial nitric oxide synthase
- GTN:
-
Glycerol trinitrate
- HO-1:
-
Heme oxygenase 1
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate, reduced form
- MCP-1:
-
Monocyte chemotactic protein 1
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- PEG-SOD:
-
Polyethylene-glycolated-superoxide dismutase
- TBP:
-
TATA box binding protein
- VCAM-1:
-
Vascular cell adhesion protein 1
References
Abdel Malik R, Zippel N, Fromel T, Heidler J, Zukunft S, Walzog B, Ansari N, Pampaloni F, Wingert S, Rieger MA, Wittig I, Fisslthaler B, Fleming I (2017) AMP-activated protein kinase alpha2 in neutrophils regulates vascular repair via hypoxia-inducible factor-1alpha and a network of proteins affecting metabolism and apoptosis. Circ Res 120:99–109. https://doi.org/10.1161/circresaha.116.309937
Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML (2006) VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 235:759–767. https://doi.org/10.1002/dvdy.20643
Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE (1999) AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443:285–289. https://doi.org/10.1016/s0014-5793(98)01705-0
Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T (2004) Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med 36:101–111. https://doi.org/10.1016/j.freeradbiomed.2003.10.012
De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL (2005) Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25:2106–2113. https://doi.org/10.1161/01.atv.0000181743.28028.57
Dong Y, Zhang M, Liang B, **e Z, Zhao Z, Asfa S, Choi HC, Zou MH (2010) Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation 121:792–803. https://doi.org/10.1161/circulationaha.109.900928
Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, Izon D, Honeyman J, Chen ZP, van Denderen BJ, Kemp BE, Steinberg GR (2011) Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Investig 121:4903–4915. https://doi.org/10.1172/jci58577
Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA (2002) Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105:1567–1572. https://doi.org/10.1161/01.cir.0000012543.55874.47
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204:2449–2460. https://doi.org/10.1084/jem.20070657
He C, Li H, Viollet B, Zou MH, **e Z (2015) AMPK suppresses vascular inflammation in vivo by inhibiting signal transducer and activator of transcription-1. Diabetes 64:4285–4297. https://doi.org/10.2337/db15-0107
Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:E14–E22. https://doi.org/10.1161/01.res.88.2.e14
Jansen T, Kroller-Schon S, Schonfelder T, Foretz M, Viollet B, Daiber A, Oelze M, Brandt M, Steven S, Kvandova M, Kalinovic S, Lagrange J, Keaney JF Jr, Munzel T, Wenzel P, Schulz E (2018) Alpha1AMPK deletion in myelomonocytic cells induces a pro-inflammatory phenotype and enhances angiotensin II-induced vascular dysfunction. Cardiovasc Res 114:1883–1893. https://doi.org/10.1093/cvr/cvy172
Kadlec AO, Beyer AM, Ait-Aissa K, Gutterman DD (2016) Mitochondrial signaling in the vascular endothelium: beyond reactive oxygen species. Basic Res Cardiol 111:26. https://doi.org/10.1007/s00395-016-0546-5
Kleschyov AL, Mollnau H, Oelze M, Meinertz T, Huang Y, Harrison DG, Munzel T (2000) Spin trap** of vascular nitric oxide using colloid Fe(II)-diethyldithiocarbamate. Biochem Biophys Res Commun 275:672–677. https://doi.org/10.1006/bbrc.2000.3361
Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA (2001) Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med 193:741–754. https://doi.org/10.1084/jem.193.6.741
Kossmann S, Hu H, Steven S, Schonfelder T, Fraccarollo D, Mikhed Y, Brahler M, Knorr M, Brandt M, Karbach SH, Becker C, Oelze M, Bauersachs J, Widder J, Munzel T, Daiber A, Wenzel P (2014) Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. J Biol Chem 289:27540–27550. https://doi.org/10.1074/jbc.m114.604231
Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, **a N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T, Daiber A (2014) Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 20:247–266. https://doi.org/10.1089/ars.2012.4953
Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone MA Jr, Ertl G, Huang PL (2004) Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol 286:C1195–C1202. https://doi.org/10.1152/ajpcell.00546.2002
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mancini SJ, White AD, Bijland S, Rutherford C, Graham D, Richter EA, Viollet B, Touyz RM, Palmer TM, Salt IP (2017) Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol Cell Endocrinol 440:44–56. https://doi.org/10.1016/j.mce.2016.11.010
Mangalam AK, Rattan R, Suhail H, Singh J, Hoda MN, Deshpande M, Fulzele S, Denic A, Shridhar V, Kumar A, Viollet B, Rodriguez M, Giri S (2016) AMP-activated protein kinase suppresses autoimmune central nervous system disease by regulating M1-type macrophage-Th17 axis. J Immunol 197:747–760. https://doi.org/10.4049/jimmunol.1501549
Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML (2006) VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn 235:3413–3422. https://doi.org/10.1002/dvdy.20982
Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, Viollet B, Chazaud B (2013) AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab 18:251–264. https://doi.org/10.1016/j.cmet.2013.06.017
Munzel T, Giaid A, Kurz S, Stewart DJ, Harrison DG (1995) Evidence for a role of endothelin-1 and protein-kinase-C in nitroglycerin tolerance. Proc Natl Acad Sci USA 92:5244–5248. https://doi.org/10.1073/pnas.92.11.5244
Oelze M, Daiber A, Brandes RP, Hortmann M, Wenzel P, Hink U, Schulz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, Li H, Forstermann U, Munzel T (2006) Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 48:677–684. https://doi.org/10.1161/01.hyp.0000239207.82326.29
Rutherford C, Speirs C, Williams JJ, Ewart MA, Mancini SJ, Hawley SA, Delles C, Viollet B, Costa-Pereira AP, Baillie GS, Salt IP, Palmer TM (2016) Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Sci Signal 9:ra109. https://doi.org/10.1126/scisignal.aaf8566
Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF Jr, Wegener G, Lackner K, Munzel T, Viollet B, Schulz E (2011) Alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arterioscler Thromb Vasc Biol 31:560–566. https://doi.org/10.1161/atvbaha.110.219543
Schulz E, Anter E, Keaney JF Jr (2004) Oxidative stress, antioxidants, and endothelial function. Curr Med Chem 11:1093–1104. https://doi.org/10.2174/0929867043365369
Schulz E, Anter E, Zou MH, Keaney JF Jr (2005) Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation 111:3473–3480. https://doi.org/10.1161/circulationaha.105.546812
Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF Jr (2008) Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation 118:1347–1357. https://doi.org/10.1161/circulationaha.108.784298
Shimasaki Y, Pan N, Messina LM, Li C, Chen K, Liu L, Cooper MP, Vita JA, Keaney JF Jr (2013) Uncoupling protein 2 impacts endothelial phenotype via p53-mediated control of mitochondrial dynamics. Circ Res 113:891–901. https://doi.org/10.1161/circresaha.113.301319
Singer BD, Mock JR, D’Alessio FR, Aggarwal NR, Mandke P, Johnston L, Damarla M (2016) Flow-cytometric method for simultaneous analysis of mouse lung epithelial, endothelial, and hematopoietic lineage cells. Am J Physiol Lung Cell Mol Physiol 310:L796–L801. https://doi.org/10.1152/ajplung.00334.2015
Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM (1999) Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation 100:1223–1229. https://doi.org/10.1161/01.cir.100.11.1223
Wang XX, Wang XL, Tong MM, Gan L, Chen H, Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, Zhu Y, Li YX, He JH, Wang M, Jiang W (2016) SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3alpha-dependent antioxidant defense mechanisms. Basic Res Cardiol 111:13. https://doi.org/10.1007/s00395-016-0531-z
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T (2011) Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124:1370–1381. https://doi.org/10.1161/circulationaha.111.034470
Wiemer G, Linz W, Hatrik S, Scholkens BA, Malinski T (1997) Angiotensin-converting enzyme inhibition alters nitric oxide and superoxide release in normotensive and hypertensive rats. Hypertension 30:1183–1190. https://doi.org/10.1161/01.hyp.30.5.1183
**ng J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH (2013) Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol 182:1021–1030. https://doi.org/10.1016/j.ajpath.2012.11.022
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:145–153. https://doi.org/10.1056/nejm200001203420301
Acknowledgements
We thank Bettina Mros, Angelica Karpi, Jessica Rudolph and Jörg Schreiner for excellent technical assistance. Data of the present study are part of the medical thesis of Thi Lan P. Tran. This work was supported by the European Commission integrated project (LSHM-CT-2004-005272/exgenesis) to B.V., by the German Research Foundation (DFG SCHU 1486/4-1 to E.S. and DFG WE4361/7-1 to P.W.) and by the Federal Ministry of Education and Research (BMBF 01EO1503).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kröller-Schön, S., Jansen, T., Tran, T.L.P. et al. Endothelial α1AMPK modulates angiotensin II-mediated vascular inflammation and dysfunction. Basic Res Cardiol 114, 8 (2019). https://doi.org/10.1007/s00395-019-0717-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0717-2