Abstract
Objectives
To investigate the potential of decreasing the number of scans and associated radiation exposure involved in CT liver perfusion (CTLP) dynamic studies for hepatocellular carcinoma (HCC) assessment.
Methods
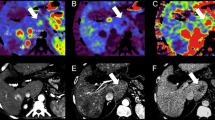
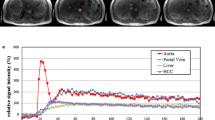
Twenty-four CTLP image datasets of patients with HCC were retrospectively analyzed. All examinations were performed on a modern CT system using a standard acquisition protocol involving 35 scans with 1.7 s interval. A deconvolution-based or a standard algorithm was employed to compute ten perfusion parametric maps. 3D ROIs were positioned on 33 confirmed HCCs and non-malignant parenchyma. Analysis was repeated for two subsampled datasets generated from the original dataset by including only the (a) 18 odd-numbered scans with 3.4 s interval and (b) 18 first scans with 1.7 s interval. Standard and modified datasets were compared regarding the (a) accuracy of calculated perfusion parameters, (b) power of parametric maps to discriminate HCCs from liver parenchyma, and (c) associated radiation exposure.
Results
When the time interval between successive scans was doubled, perfusion parameters of HCCs were found unaffected (p > 0.05) and the discriminating efficiency of parametric maps was preserved (p < 0.05). In contrast, significant differences were found for all perfusion parameters of HCCs when acquisition duration was reduced to half (p < 0.05), while the discriminating efficiency of four parametric maps was significantly deteriorated (p < 0.05). Modified CTLP acquisition protocols were found to involve 48.5% less patient exposure.
Conclusions
Doubling the interscan time interval may considerably reduce radiation exposure from CTLP studies performed for HCC evaluation without affecting the diagnostic efficiency of perfusion maps generated with either standard or deconvolution-based mathematical model.
Key Points
• CT liver perfusion for HCC diagnosis/assessment is not routinely used in clinical practice mainly due to the associated high radiation exposure.
• Two alternative acquisition protocols involving 18 scans of the liver were compared with the standard 35-scan protocol.
• Increasing the time interval between successive scans to 3.4 s was found to preserve the accuracy of computed perfusion parameters derived with a standard or a deconvolution-based model and to reduce radiation exposure by 48.5%.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the ROC curve
- BF:
-
Blood flow
- BV:
-
Blood volume
- CTLP:
-
Computed tomography liver perfusion
- DLP:
-
Dose length product
- DSA:
-
Digital subtraction angiography
- EASL:
-
European Association for the Study of the Liver
- ED:
-
Effective dose
- HaBF:
-
Hepatic arterial blood flow
- HAF:
-
Hepatic arterial fraction
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- IRF:
-
Impulse residue function
- IRF-T0:
-
Contrast arrival delay
- IQR:
-
Interquartile range
- MSI:
-
Mean slope of increase
- MTT:
-
Mean transit time
- PEI:
-
Positive enhancement integral
- PS:
-
Permeability surface
- REML:
-
Restricted maximum likelihood
- Tmax:
-
Transit time to impulse residue peak
- TTP:
-
Time to peak
References
Liapi E, Mahesh M, Sahani DV (2015) Is CT perfusion ready for liver cancer treatment evaluation? J Am Coll Radiol 12:111–113
Ippolito D, Pecorelli A, Querques G et al (2019) Dynamic computed tomography perfusion imaging: complementary diagnostic tool in hepatocellular carcinoma assessment from diagnosis to treatment follow-up. Acad Radiol 26:1675–1685
Kim SH, Kamaya A, Willmann JK (2014) CT perfusion of the liver: principles and applications in oncology. Radiology 272:322–344
Miles KA, Hayball MP, Dixon AK (1993) Functional images of hepatic perfusion obtained with dynamic CT. Radiology 188:405–411
Materne R, Van Beers BE, Smith AM et al (2000) Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond) 99:517–525
Lee TY, Purdie TG, Stewart E (2003) CT imaging of angiogenesis. Q J Nucl Med 47:171–87
Miles KA, Lee TY, Goh V et al (2012) Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur Radiol 22:1430–1441
Perisinakis K, Tzedakis A, Pouli S, Spanakis K, Hatzidakis A, Damilakis J (2019) Comparison of patient dose from routine multi-phase and dynamic liver perfusion CT studies taking into account the effect of iodinated contrast administration. Eur J Radiol 110:39–44
Topcuoglu OM, Karçaaltincaba M, Akata D, Özmen MN (2016) Reproducibility and variability of very low dose hepatic perfusion CT in metastatic liver disease. Diagn Interv Radiol 22:495–500
Enjilela E, Lee TY, Hsieh J et al (2017) Ultra-low-dose sparse-view quantitative CT liver perfusion imaging. Tomography 3:175–179
**ao Y, Liu P, Liang Y et al (2019) STIR-net: deep spatial-temporal image restoration net for radiation reduction in CT perfusion. Front Neurol 10:647
Brix G, Lechel U, Nekolla E, Griebel J, Becker C (2015) Radiation protection issues in dynamic contrast-enhanced (perfusion) computed tomography. Eur J Radiol 84:2347–2358
Klotz E, Haberland U, Glatting G et al (2015) Technical prerequisites and imaging protocols for CT perfusion imaging in oncology. Eur J Radiol 84:2359–2367
Goh V, Dattani M, Farwell J et al (2011) Radiation dose from volumetric helical perfusion CT of the thorax, abdomen or pelvis. Eur Radiol 21:974–981
Ng CS, Chandler AG, Wei W et al (2013) Effect of sampling frequency on perfusion values in perfusion CT of lung tumors. AJR Am J Roentgenol 200:W155–W162
Ng CS, Hobbs BP, Wei W et al (2015) Effect on perfusion values of sampling interval of computed tomographic perfusion acquisitions in neuroendocrine liver metastases and normal liver. J Comput Assist Tomogr 39:373–382
European Association For The Study Of The Liver (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Hatzidakis A, Perisinakis K, Kalarakis G et al (2018) Perfusion-CT analysis for assessment of hepatocellular carcinoma lesions: diagnostic value of different perfusion maps. Acta Radiol 284185118791200
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
van Ommen F, Kauw F, Bennink E, Dankbaar JW, Viergever MA, de Jong HWAM (2019) Effect of prolonged acquisition intervals for CT-perfusion analysis methods in patients with ischemic stroke. Med Phys 46:3156–3164
Karwacki GM, Vögele S, Blackham KA (2019) Dose reduction in perfusion CT in stroke patients by lowering scan frequency does not affect automatically calculated infarct core volumes. J Neuroradiol 46:351–358
Kambadakone AR, Sharma A, Catalano OA, Hahn PF, Sahani DV (2011) Protocol modifications for CT perfusion (CTp) examinations of abdomen-pelvic tumors: impact on radiation dose and data processing time. Eur Radiol 21:1293–1300
Goh V, Halligan S, Hugill JA, Gartner L, Bartram CI (2005) Quantitative colorectal cancer perfusion measurement using dynamic contrast-enhanced multidetector-row computed tomography: effect of acquisition time and implications for protocols. J Comput Assist Tomogr 29:59–63
Ng CS, Hobbs BP, Chandler AG et al (2013) Metastases to the liver from neuroendocrine tumors: effect of duration of scan acquisition on CT perfusion values. Radiology 269:758–767
Bayle M, Clerc-Urmes I, Ayav A et al (2019) Computed tomographic perfusion with 160-mm coverage: comparative analysis of hepatocellular carcinoma treated by two transarterial chemoembolization courses relative to magnetic resonance imaging findings. Abdom Radiol (NY) 44:85–94
Kurucay M, Kloth C, Kaufmann S et al (2017) Multiparametric imaging for detection and characterization of hepatocellular carcinoma using gadoxetic acid-enhanced MRI and perfusion-CT: which parameters work best? Cancer Imaging 17:18
Gawlitza J, Haubenreisser H, Meyer M et al (2016) Comparison of organ-specific-radiation dose levels between 70 kVp perfusion CT and standard tri-phasic liver CT in patients with hepatocellular carcinoma using a Monte-Carlo-simulation-based analysis platform. Eur J Radiol Open 3:95–99
Lee DH, Lee JM, Klotz E, Han JK (2016) Multiphasic dynamic computed tomography evaluation of liver tissue perfusion characteristics using the dual maximum slope model in patients with cirrhosis and hepatocellular carcinoma: a feasibility study. Invest Radiol 51:430–434
Fischer MA, Kartalis N, Grigoriadis A et al (2015) Perfusion computed tomography for detection of hepatocellular carcinoma in patients with liver cirrhosis. Eur Radiol 25:3123–3132
Cros M, Geleijns J, Joemai RMS, Salvado M (2016) Perfusion CT of the brain and liver and of lung tumors: use of Monte Carlo simulation for patient dose estimation for examinations with a cone-beam 320-MDCT scanner. AJR Am J Roentgenol 206:129–135
Brehmer K, Brismar TB, Morsbach F et al (2018) Triple arterial phase CT of the liver with radiation dose equivalent to that of single arterial phase CT: initial experience. Radiology 289:111–118
Bevilacqua A, Malavasi S, Vilgrain V (2019) Liver CT perfusion: which is the relevant delay that reduces radiation dose and maintains diagnostic accuracy? Eur Radiol 29:6550–6558
Thaiss WM, Haberland U, Kaufmann S et al (2019) Dose optimization of perfusion-derived response assessment in hepatocellular carcinoma treated with transarterial chemoembolization: comparison of volume perfusion CT and iodine concentration. Acad Radiol 26:1154–1163
Kanda T, Yoshikawa T, Ohno Y et al (2012) CT hepatic perfusion measurement: comparison of three analytic methods. Eur J Radiol 81:2075–2079
Hatzidakis A, Perisinakis K, Kalarakis G et al (2019) Perfusion-CT analysis for assessment of hepatocellular carcinoma lesions: diagnostic value of different perfusion maps. Acta Radiol 60:561–568
Ippolito D, Capraro C, Casiraghi A, Cestari C, Sironi S (2012) Quantitative assessment of tumour associated neovascularisation in patients with liver cirrhosis and hepatocellular carcinoma: role of dynamic-CT perfusion imaging. Eur Radiol 22:803–811
Fournier LS, Cuenod CA, de Bazelaire C et al (2004) Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol 14:2125–2133
Sahani DV, Holalkere NS, Mueller PR, Zhu AX (2007) Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue - initial experience. Radiology 243:736–743
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Adam Hatzidakis.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalarakis, G., Perisinakis, K., Akoumianakis, E. et al. CT liver perfusion in patients with hepatocellular carcinoma: can we modify acquisition protocol to reduce patient exposure?. Eur Radiol 31, 1410–1419 (2021). https://doi.org/10.1007/s00330-020-07206-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07206-9