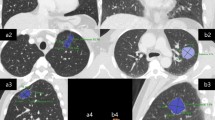
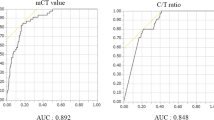
Abstract
Objectives
Lung adenocarcinoma frequently manifests as subsolid nodules, and the solid portion and ground-glass-opacity (GGO) portion on CT have different prognostic significance. Therefore, current T descriptor, defined as the whole tumour diameter without discrimination between solid and GGO, is insufficient. We aimed to determine the prognostic significance of solid tumour size and attempt to include prognostic factors such as tumour disappearance rate (TDR) on CT and SUVmax on PET/CT.
Methods
Five hundred and ninety-five patients with completely resected lung adenocarcinoma were analyzed. We developed a nomogram using whole tumour size, TDR, and SUVmax. External validation was performed in another 102 patients.
Results
In patients with tumours measuring ≤2 cm and >2 to 3 cm, disease free survival (DFS) was significantly associated with solid tumour size (P < 0.001), but not with whole tumour size (P = 0.052). Developed nomogram was significantly superior to the conventional T stage (area under the curve of survival ROC; P = 0.013 by net reclassification improvement) in stratification of patient survival. In the external validation group, significant difference was noted in DFS according to proposed T stage (P = 0.009).
Conclusions
Nomogram-based T descriptors provide better prediction of survival and assessment of individual risks than conventional T descriptors.
Key points
• Current measurement of whole tumour diameter including ground-glass opacity is insufficient
• TDR enables differentiation between invasive solid portion and non-invasive GGO portion
• SUVmax demonstrates the biological aggressiveness of the tumour
• We developed a nomogram using whole tumour size, TDR, and SUVmax
• Nomogram-based clinical T descriptors provide better prediction of survival



Similar content being viewed by others
Abbreviations
- AIS:
-
Adenocarcinoma in situ
- DFS:
-
Disease-free survival
- FDG:
-
Fluorodeoxyglucose
- GGO:
-
Ground-glass opacity
- MIA:
-
Minimally invasive adenocarcinoma
- NRI:
-
Net reclassification improvement
- OS:
-
Overall survival
- TDR:
-
Tumour disappearance rate
References
Aberle DR, Adams AM, Berg CD et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Chen F, Cole P, Bina WF (2007) Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973-2002. Cancer Epidemiol Biomarkers Prev 16:2724–2729
Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA (2011) Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 6:1496–1504
Kuriyama K, Seto M, Kasugai T et al (1999) Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol 173:465–469
Aoki T, Tomoda Y, Watanabe H et al (2001) Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 220:803–809
Higashiyama M, Kodama K, Yokouchi H et al (1999) Prognostic value of bronchiolo-alveolar carcinoma component of small lung adenocarcinoma. Ann Thorac Surg 68:2069–2073
Lee HY, Lee KS (2011) Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 26:106–118
Yoshizawa A, Motoi N, Riely GJ et al (2011) Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 24:653–664
Okada M, Nishio W, Sakamoto T et al (2004) Correlation between computed tomographic findings, bronchioloalveolar carcinoma component, and biologic behavior of small-sized lung adenocarcinomas. J Thorac Cardiovasc Surg 127:857–861
Okada M, Nishio W, Sakamoto T, Uchino K, Tsubota N (2003) Discrepancy of computed tomographic image between lung and mediastinal windows as a prognostic implication in small lung adenocarcinoma. Ann Thorac Surg 76:1828–1832, discussion 1832
Austin JH, Garg K, Aberle D et al (2013) Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 266:62–71
Greaves SM, Brown K, Garon EB, Garon BL (2011) The new staging system for lung cancer: imaging and clinical implications. J Thorac Imaging 26:119–131
Murakawa T, Konoeda C, Ito T et al (2013) The ground glass opacity component can be eliminated from the T-factor assessment of lung adenocarcinoma. Eur J Cardiothorac Surg 43:925–932
Nakamura S, Fukui T, Taniguchi T et al (2013) Prognostic impact of tumor size eliminating the ground glass opacity component: modified clinical T descriptors of the tumor, node, metastasis classification of lung cancer. J Thorac Oncol 8:1551–1557
Tsutani Y, Miyata Y, Nakayama H et al (2013) Solid tumor size on high-resolution computed tomography and maximum standardized uptake on positron emission tomography for new clinical T descriptors with T1 lung adenocarcinoma. Ann Oncol 24:2376–2381
Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N (2006) Radical sublobar resection for small-sized non–small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg 132:769–775
Hubner KF, Buonocore E, Singh SK, Gould HR, Cotten DW (1995) Characterization of chest masses by FDG positron emission tomography. Clin Nucl Med 20:293–298
Yap CS, Schiepers C, Fishbein MC, Phelps ME, Czernin J (2002) FDG-PET imaging in lung cancer: how sensitive is it for bronchioloalveolar carcinoma? Eur J Nucl Med Mol Imaging 29:1166–1173
Tsutani Y, Miyata Y, Nakayama H et al (2014) Segmentectomy for clinical stage IA lung adenocarcinoma showing solid dominance on radiology. Eur J Cardiothorac Surg 46:637–642
Tsutani Y, Miyata Y, Nakayama H et al (2012) Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 143:607–612
Lee HY, Choi YL, Lee KS et al (2014) Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol 202:W224–W233
Lee HY, Han J, Lee KS et al (2009) Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer 66:379–385
Nakayama H, Yamada K, Saito H et al (2007) Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 84:1675–1679
Lee HY, Jeong JY, Lee KS et al (2013) Histopathology of lung adenocarcinoma based on new IASLC/ATS/ERS classification: prognostic stratification with functional and metabolic imaging biomarkers. J Magn Reson Imaging 38:905–913
Lester SC (2006) Manual of surgical pathology, 2nd edn. Elsevier Churchill Livingstone, New York
Travis WD, Brambilla E, Noguchi M et al (2011) International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285
Vickers AJ, Pepe M (2014) Does the net reclassification improvement help us evaluate models and markers? Ann Intern Med 160:136–137
Hashizume T, Yamada K, Okamoto N et al (2008) Prognostic significance of thin-section CT scan findings in small-sized lung adenocarcinoma. Chest 133:441–447
Matsuguma H, Yokoi K, Anraku M et al (2002) Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: a predictor of lymph node metastasis. J Thorac Cardiovasc Surg 124:278–284
Takahashi M, Shigematsu Y, Ohta M, Tokumasu H, Matsukura T, Hirai T (2014) Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 147:54–59
Downey RJ, Akhurst T, Gonen M et al (2004) Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 22:3255–3260
Higashi K, Ueda Y, Arisaka Y et al (2002) 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 43:39–45
van Rens MT, de la Riviere AB, Elbers HR, van Den Bosch JM (2000) Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest 117:374–379
Goldstraw P, Crowley J, Chansky K et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage grou**s in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2:706–714
Kadota K, Colovos C, Suzuki K et al (2012) FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Ann Surg Oncol 19:3598–3605
Acknowledgments
The scientific guarantor of this publication is Ho Yun Lee. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding authors
Additional information
So Hee Song and Joong Hyun Ahn contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Flow chart of participant enrolment. (GIF 11 kb)
Supplementary Figure 2
Waterfall plot demonstrates distribution of T status in the training group and external validation group. (GIF 27 kb)
Supplementary Figure 3
Three lung adenocarcinomas of different size and internal composition. (a) 2.2 cm sized entirely solid lung adenocarcinoma. (b) 2.2 cm sized part-solid lung adenocarcinoma with 1 cm of solid portion. (c) 1 cm sized entirely solid lung adenocarcinoma. (GIF 575 kb)
Supplementary Table 1
(DOCX 16 kb)
Supplementary Table 2
(DOCX 18 kb)
Supplementary Table 3
(DOCX 25 kb)
Supplementary Table 4
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Song, S.H., Ahn, J.H., Lee, H.Y. et al. Prognostic impact of nomogram based on whole tumour size, tumour disappearance ratio on CT and SUVmax on PET in lung adenocarcinoma. Eur Radiol 26, 1538–1546 (2016). https://doi.org/10.1007/s00330-015-4029-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4029-0