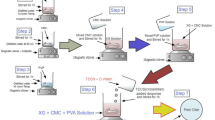
Abstract
Over the time, hydrogels have emerged as one of the most potential candidates for drug delivery and tissue engineering systems due to their swellable and porous nature. Fabrication process of hydrogel requires addition of crosslinkers. Various chemical (e.g., crosslinking by chemical reaction of complementary groups, polymer–polymer crosslinking, high energy irradiation and enzyme incorporation) and physical (e.g., charge interactions, crystallization and stereocomplex formation) approaches have been employed for crosslinking hydrogels. Majority of the conventionally employed crosslinkers are toxic in nature and unfavorable for use. Moreover, they have poor water solubility and low biodegradation rate. Various natural (e.g., vanillin, citric acid, gallic acid, ferulic acid and genipin) and synthetic (e.g., polymerizable polyphosphate, 1,2,3,4-butanetetracarboxylic dianhydride and 2-chloro-1-methylpyrinium iodide) novel crosslinking agents have been developed to overcome these limitations and to produce hydrogels with good mechanical properties. Furthermore, novel non-toxic crosslinkers are being introduced for modulating release characteristics and attaining controlled drug release profile of hydrogels made up of highly soluble and erodible polymers. Considering the drawbacks of conventional crosslinkers, there is a need to search for more biocompatible and biodegradable novel polymers to attain safe and efficient hydrogel formulations.




Similar content being viewed by others
References
Chen G, Tang W, Wang X, Zhao X, Chen C, Zhu Z (2019) Applications of hydrogels with special physical properties in biomedicine. Polymers 11(9):1–17
Arshad MS, Zahra AT, Zafar S, Zaman H, Akhtar A, Ayaz MM, Kucuk I, Maniruzzaman M, Chang M-W, Ahmad Z (2021) Antibiofilm effects of macrolide loaded microneedle patches: prospects in healing infected wounds. Pharm Res 38(1):165–177
Arshad MS, Zafar S, Zahra AT, Zaman MH, Akhtar A, Kucuk I, Farhan M, Chang M-W, Ahmad Z (2021) Fabrication and characterisation of self-applicating heparin sodium microneedle patches. J Drug Target 29(1):60–68
Khan S, Ullah A, Ullah K, Rehman N-u (2016) Insight into hydrogels. Des Monomers Polym 19(5):456–478
Ahmed EM (2015) Hydrogel: Preparation, characterization, and applications: a review. J Adv Res 6(2):105–121
Akhtar MF, Hanif M, Ranjha NM (2016) Methods of synthesis of hydrogels… a review. Saudi Pharm J 24(5):554–559
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49(8):1993–2007
Parhi R (2017) Cross-linked hydrogel for pharmaceutical applications: a review. Adv Pharm Bull 7(4):515–530
Mondal S, Das S, Nandi AK (2020) A review on recent advances in polymer and peptide hydrogels. Soft Matter 16(6):1404–1454
Kevin J, D'Emilio E, Cranston ED, Geiger T, Nyström G (2020) Dual physically and chemically crosslinked regenerated cellulose–Gelatin composite hydrogels towards art restoration. Carbohydr Polym 234(1):115885
Zhang X, Zhang R, Wu S, Sun Y, Yang H, Lin B (2020) Physically and chemically dual-crosslinked hydrogels with superior mechanical properties and self-healing behavior. New J Chem 44(23):9903–9911
Hellio D, Djabourov M (2006) Physically and chemically crosslinked gelatin gels. Macromol Symp 241(1):23–27
Bashir S, Hina M, Iqbal J, Rajpar A, Mujtaba M, Alghamdi N, Wageh S, Ramesh K, Ramesh S (2020) Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers 12(11):1–60
Hennink WE, van Nostrum CF (2012) Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 64(1):223–236
Naeem F, Khan S, Jalil A, Ranjha NM, Riaz A, Haider MS, Sarwar S, Saher F, Afzal S (2017) pH responsive cross-linked polymeric matrices based on natural polymers: effect of process variables on swelling characterization and drug delivery properties. BioImpacts 7(3):177–192
Hu W, Wang Z, **ao Y, Zhang S, Wang J (2019) Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci 7(3):843–855
Peppas NA, Berner RE Jr (1980) Proposed method of intracopdal injection and gelation of poly (vinyl alcohol) solution in vocal cords: polymer considerations. Biomaterials 1(3):158–162
Dai W, Barbari T (1999) Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly (vinyl alcohol). J Membr Sci 156(1):67–79
Tabata Y, Ikada Y (1989) Synthesis of gelatin microspheres containing interferon. Pharm Res 6(5):422–427
Luo Y, Kirker KR, Prestwich GD (2000) Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release 69(1):169–184
Draye J-P, Delaey B, Van de Voorde A, Van Den Bulcke A, Bogdanov B, Schacht E (1998) In vitro release characteristics of bioactive molecules from dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials 19(1–3):99–107
Elbert D, Lutolf M, Pratt A, Halstenberg S, Hubbell J (2001) Protein release from PEG hydrogels that are similar to ideal Flory-Rehner Networks. Controlled Release Bioact Mater 28(1):987–988
de Nooy AE, Capitani D, Masci G, Crescenzi V (2000) Ionic polysaccharide hydrogels via the Passerini and Ugi multicomponent condensations: synthesis, behavior and solid-state NMR characterization. Biomacromol 1(2):259–267
Ichi T, Watanabe J, Ooya T, Yui N (2001) Controllable erosion time and profile in poly (ethylene glycol) hydrogels by supramolecular structure of hydrolyzable polyrotaxane. Biomacromolecules 2(1):204–210
Tan H, Chu CR, Payne KA, Marra KG (2009) Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 30(13):2499–2506
Lee KY, Alsberg E, Mooney DJ (2001) Degradable and injectable poly (aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res 56(2):228–233
Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA (2001) Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release 76(1–2):11–25
Giammona G, Pitarresi G, Cavallaro G, Spadaro G (1999) New biodegradable hydrogels based on an acryloylated polyaspartamide cross-linked by gamma irradiation. J Biomater Sci Polym Ed 10(9):969–987
Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J (2007) Novel in situ forming, degradable dextran hydrogels by Michael addition chemistry: synthesis, rheology, and degradation. Macromolecules 40(4):1165–1173
Bulpitt P, Aeschlimann D (1999) New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res 47(2):152–169
Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS (2007) The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 28(6):975–983
Moerkerke R, Meeussen F, Koningsveld R, Berghmans H, Mondelaers W, Schacht E, Dušek K, Šolc K (1998) Phase transitions in swollen networks. 3. Swelling behavior of radiation cross-linked poly (vinyl methyl ether) in water. Macromolecules 31(7):2223–2229
Arndt K-F, Schmidt T, Reichelt R (2001) Thermo-sensitive poly (methyl vinyl ether) micro-gel formed by high energy radiation. Polymer 42(16):6785–6791
Jeong J-O, Park J-S, Kim EJ, Jeong S-I, Lee JY, Lim Y-M (2020) Preparation of radiation cross-linked poly (Acrylic acid) hydrogel containing metronidazole with enhanced antibacterial activity. Int J Mol Sci 21(1):1–14
Jeong J-O, Park J-S, Kim Y-A, Yang S-J, Jeong S-I, Lee J-Y, Lim Y-M (2020) Gamma ray-induced polymerization and cross-linking for optimization of PPy/PVP hydrogel as biomaterial. Polymers 12(1):1–11
Mitsui H, Hosoi F, Kagiya T (1973) γ-radiation-induced cross-linking of polyethylene. Polym J 4(1):79–86
Rimdusit S, Somsaeng K, Kewsuwan P, Jubsilp C, Tiptipakorn S (2012) Comparison of gamma radiation crosslinking and chemical crosslinking on properties of methylcellulose hydrogel. Eng J 16(4):15–28
Tavakol M, Dehshiri S, Vasheghani-Farahani E (2016) Electron beam irradiation crosslinked hydrogels based on tyramine conjugated gum tragacanth. Carbohydr Polym 152(1):504–509
Moghaddam RH, Dadfarnia S, Shabani AMH, Moghaddam ZH, Tavakol M (2019) Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater Sci Eng C 102(1):391–404
Călina I, Demeter M, Scărișoreanu A, Sătulu V, Mitu B (2020) One step e-beam radiation cross-linking of quaternary hydrogels dressings based on chitosan-poly (vinyl-pyrrolidone)-poly (ethylene glycol)-poly (acrylic acid). Int J Mol Sci 21(23):1–24
Suliwarno A (2014) Hydrogel based on crosslinked methylcellulose prepared by electron beam irradiation for wound dressing application. Indones J Chem 14(3):262–268
Ono K, Saito Y, Yura H, Ishikawa K, Kurita A, Akaike T, Ishihara M (2000) Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res 49(2):289–295
Xu Q, McMichael P, Creagh-Flynn J, Zhou D, Gao Y, Li X, Wang X, Wang W (2018) Double-cross-linked hydrogel strengthened by UV irradiation from a hyperbranched PEG-based trifunctional polymer. ACS Macro Lett 7(5):509–513
Shamekhi M, Jafari S, Khonakdar H, Ehsani M (2010) Preparation and characterisation of UV irradiation cross-linked LDPE/EVA blends. Plast Rubber Compos 39(10):431–436
Teixeira RS, Correa RJ, Belvino A, Nascimento RS (2013) UV Irradiation-induced crosslinking of aqueous solution of poly (ethylene oxide) with benzophenone as initiator. J Appl Polym Sci 130(4):2458–2467
Cook JP, Goodall GW, Khutoryanskaya OV, Khutoryanskiy VV (2012) Microwave-assisted hydrogel synthesis: a new method for crosslinking polymers in aqueous solutions. Macromol Rapid Commun 33(4):332–336
Larraneta E, Lutton RE, Brady AJ, Vicente-Pérez EM, Woolfson AD, Thakur RRS, Donnelly RF (2015) Microwave-assisted preparation of hydrogel-forming microneedle arrays for transdermal drug delivery applications. Macromol Mater Eng 300(6):586–595
Radwan-Pragłowska J, Piątkowski M, Janus Ł, Bogdał D, Matysek D, Čablik V (2019) Microwave-assisted synthesis and characterization of antibacterial O-crosslinked chitosan hydrogels doped with TiO2 nanoparticles for skin regeneration. Int J Polym Mater Polym Biomater 68(15):881–890
Sperinde JJ, Griffith LG (1997) Synthesis and characterization of enzymatically-cross-linked poly (ethylene glycol) hydrogels. Macromolecules 30(18):5255–5264
Yung C, Wu L, Tullman J, Payne G, Bentley W, Barbari T (2007) Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J Biomed Mater Res A 83(4):1039–1046
Kim K, Park S, Yang J-A, Jeon J-H, Bhang S, Kim B-S, Hahn S (2011) Injectable hyaluronic acid–tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater 7(2):666–674
Wu J, Su Z-G, Ma G-H (2006) A thermo-and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int J Pharm 315(1–2):1–11
Gacesa P (1988) Alginates. Carbohydr Polym 8(3):161–182
Gombotz WR, Wee S (1998) Protein release from alginate matrices. Adv Drug Deliv Rev 31(3):267–285
Goosen MF, O’Shea GM, Gharapetian HM, Chou S, Sun AM (1985) Optimization of microencapsulation parameters: semipermeable microcapsules as a bioartificial pancreas. Biotechnol Bioeng 27(2):146–150
Polk A, Amsden B, De Yao K, Peng T, Goosen M (1994) Controlled release of albumin from chitosan-alginate microcapsules. J Pharm Sci 83(2):178–185
Liu L-S, Liu S-Q, Ng SY, Froix M, Ohno T, Heller J (1997) Controlled release of interleukin-2 for tumour immunotherapy using alginate/chitosan porous microspheres. J Control Release 43(1):65–74
Andrianov AK, Payne LG, Visscher KB, Allcock HR, Langer R (1994) Hydrolytic degradation of ionically cross-linked polyphosphazene microspheres. J Appl Polym Sci 53(12):1573–1578
Chenite A, Chaput C, Wang D, Combes C, Buschmann M, Hoemann C, Leroux J, Atkinson B, Binette F, Selmani A (2000) Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 21(21):2155–2161
Hossain KS, Miyanaga K, Maeda H, Nemoto N (2001) Sol− gel transition behavior of pure ι-carrageenan in both salt-free and added salt states. Biomacromolecules 2(2):442–449
Janes KA, Fresneau MP, Marazuela A, Fabra A, MaJ A (2001) Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release 73(2–3):255–267
Yokoyama F, Masada I, Shimamura K, Ikawa T, Monobe K (1986) Morphology and structure of highly elastic poly (vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym Sci 264(7):595–601
Stenekes R, Talsma H, Hennink W (2001) Formation of dextran hydrogels by crystallization. Biomaterials 22(13):1891–1898
Zhang Y, Song M, Diao Y, Li B, Shi L, Ran R (2016) Preparation and properties of polyacrylamide/polyvinyl alcohol physical double network hydrogel. RSC Adv 6(113):112468–112476
Lim DW, Park TG (2000) Stereocomplex formation between enantiomeric PLA–PEG–PLA triblock copolymers: Characterization and use as protein-delivery microparticulate carriers. J Appl Polym Sci 75(13):1615–1623
Tsuji H (2005) Poly (lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol Biosci 5(7):569–597
Lim DW, Choi SH, Park TG (2000) A new class of biodegradable hydrogels stereocomplexed by enantiomeric oligo (lactide) side chains of poly (HEMA-g-OLA) s. Macromol Rapid Commun 21(8):464–471
De Jong S, van Dijk-Wolthuis W, Kettenes-Van Den Bosch J, Schuyl P, Hennink W (1998) Monodisperse enantiomeric lactic acid oligomers: preparation, characterization, and stereocomplex formation. Macromolecules 31(19):6397–6402
De Jong S, De Smedt S, Wahls M, Demeester J, Kettenes-van Den Bosch J, Hennink W (2000) Novel self-assembled hydrogels by stereocomplex formation in aqueous solution of enantiomeric lactic acid oligomers grafted to dextran. Macromolecules 33(10):3680–3686
De Jong S, De Smedt S, Demeester J, Van Nostrum C, Kettenes-Van Den Bosch J, Hennink W (2001) Biodegradable hydrogels based on stereocomplex formation between lactic acid oligomers grafted to dextran. J Control Release 72(1–3):47–56
Tokareva MI, Ivantsova MN, Mironov MA (2017) Heterocycles of natural origin as non-toxic reagents for cross-linking of proteins and polysaccharides. Chem Heterocycl Compd 53(1):21–35
Akakuru O, Isiuku B (2017) Chitosan hydrogels and their glutaraldehyde-crosslinked counterparts as potential drug release and tissue engineering systems-synthesis, characterization, swelling kinetics and mechanism. J Phys Chem Biophys 7(3):1–7
Tian Z, Liu W, Li G (2016) The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym Degrad Stab 130(1):264–270
Yu S, Zhang X, Tan G, Tian L, Liu D, Liu Y, Yang X, Pan W (2017) A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr polym 155(1):208–217
Martínez-Mejía G, Vázquez-Torres NA, Castell-Rodríguez A, del Río JM, Corea M, Jiménez-Juárez R (2019) Synthesis of new chitosan-glutaraldehyde scaffolds for tissue engineering using Schiff reactions. Colloids Surf A Physicochem Eng Asp 579(1):123658
Zhao Y, He M, Zhao L, Wang S, Li Y, Gan L, Li M, Xu L, Chang PR, Anderson DP (2016) Epichlorohydrin-cross-linked hydroxyethyl cellulose/soy protein isolate composite films as biocompatible and biodegradable implants for tissue engineering. ACS Appl Mater Interfaces 8(4):2781–2795
Huber T, Feast S, Dimartino S, Cen W, Fee C (2019) Analysis of the effect of processing conditions on physical properties of thermally set cellulose hydrogels. Materials 12(7):1–20
Imren D, Gümüşderelioğlu M, Güner A (2006) Synthesis and characterization of dextran hydrogels prepared with chlor-and nitrogen-containing crosslinkers. J Appl Polym Sci 102(5):4213–4221
Buhus G, Peptu C, Popa M, Desbrieres J (2009) Controlled release of water soluble antibiotics by carboxymethylcellulose-and gelatin-based hydrogels crosslinked with epichlorohydrin. Cellul Chem Technol 43(4):141–151
Singh A, Narvi S, Dutta P, Pandey N (2006) External stimuli response on a novel chitosan hydrogel crosslinked with formaldehyde. Bull Mater Sci 29(3):233–238
Dimida S, Barca A, Cancelli N, De Benedictis V, Raucci MG (2017) Demitri C (2017) Effects of genipin concentration on cross-linked chitosan scaffolds for bone tissue engineering: structural characterization and evidence of biocompatibility features. Int J Polym Sci 1:1–8
Heimbuck AM, Priddy-Arrington TR, Padgett ML, Llamas CB, Barnett HH, Bunnell BA, Caldorera-Moore ME (2019) Development of responsive chitosan–genipin hydrogels for the treatment of wounds. ACS Appl Biol Mater 2(7):2879–2888
Heris HK, Latifi N, Vali H, Li N, Mongeau L (2015) Investigation of chitosan-glycol/glyoxal as an injectable biomaterial for vocal fold tissue engineering. Procedia Eng 110(1):143–150
Bhumkar DR, Pokharkar VB (2006) Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: a technical note. AAPS PharmSciTech 7(2):E138–E143
Mi F-L, Sung H-W, Shyu S-S, Su C-C, Peng C-K (2003) Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer 44(21):6521–6530
Jimtaisong A, Saewan N (2018) Plant-derived polyphenols as potential cross-linking agents for methylcellulose-chitosan biocomposites. Solid State Phenom 283(1):140–146
Iwasaki Y, Nakagawa C, Ohtomi M, Ishihara K, Akiyoshi K (2004) Novel biodegradable polyphosphate cross-linker for making biocompatible hydrogel. Biomacromolecules 5(3):1110–1115
Poursamar SA, Lehner AN, Azami M, Ebrahimi-Barough S, Samadikuchaksaraei A, Antunes APM (2016) The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mater Sci Eng C 63(1):1–9
Pluda S, Pavan M, Galesso D, Guarise C (2016) Hyaluronic acid auto-crosslinked polymer (ACP): Reaction monitoring, process investigation and hyaluronidase stability. Carbohydr Res 433(1):47–53
Garnica-Palafox I, Sánchez-Arévalo F (2016) Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr polym 151(1):1073–1081
Kono H, Fujita S (2012) Biodegradable superabsorbent hydrogels derived from cellulose by esterification crosslinking with 1, 2, 3, 4-butanetetracarboxylic dianhydride. Carbohydr Polym 87(4):2582–2588
Erikci S, Mundinger P, Boehm H (2020) Small physical cross-linker facilitates hyaluronan hydrogels. Molecules 25(18):1–13
Homenick CM, de Silveira G, Sheardown H, Adronov A (2011) Pluronics as crosslinking agents for collagen: ovel amphiphilic hydrogels. Polym Int 60(3):458–465
Kasák P, Kroneková Z, Krupa I, Lacík I (2011) Zwitterionic hydrogels crosslinked with novel zwitterionic crosslinkers: Synthesis and characterization. Polymer 52(14):3011–3020
Hu X, Lu L, Xu C, Li X (2015) Mechanically tough biomacromolecular IPN hydrogel fibers by enzymatic and ionic crosslinking. Int J Biol Macromol 72(1):403–409
Zhang H, Huang X, Jiang J, Shang S, Song Z (2017) Hydrogels with high mechanical strength cross-linked by a rosin-based crosslinking agent. RSC Adv 7(67):42541–42548
Li L, Zhao J, Sun Y, Yu F, Ma J (2019) Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem Eng J 372(1):1091–1103
Li X, Qin H, Zhang X, Guo Z (2019) Triple-network hydrogels with high strength, low friction and self-healing by chemical-physical crosslinking. J Colloid Interface Sci 556(1):549–556
Subramanian K, Vijayakumar V (2013) Evaluation of isophorone diisocyanate crosslinked gelatin as a carrier for controlled delivery of drugs. Polym Bull 70(3):733–753
Sarıyer S, Duranoğlu D, Doğan Ö, Küçük İ (2020) pH-responsive double network alginate/kappa-carrageenan hydrogel beads for controlled protein release: Effect of pH and crosslinking agent. J Drug Deliv Sci Technol 56(1):101551
Song Y, Nagai N, Saijo S, Kaji H, Nishizawa M, Abe T (2018) In situ formation of injectable chitosan–gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater Sci Eng C 88(1):1–12
Mahdavinia GR, Karimi MH, Soltaniniya M, Massoumi B (2019) In vitro evaluation of sustained ciprofloxacin release from κ-carrageenan-crosslinked chitosan/hydroxyapatite hydrogel nanocomposites. Int J Biol Macromol 126(1):443–453
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zafar, S., Hanif, M., Azeem, M. et al. Role of crosslinkers for synthesizing biocompatible, biodegradable and mechanically strong hydrogels with desired release profile. Polym. Bull. 79, 9199–9219 (2022). https://doi.org/10.1007/s00289-021-03956-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03956-8