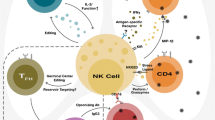
Abstract
Natural killer (NK) cells play an important role in virus control during infection. Many viruses have developed mechanisms for subversion of NK cell responses. Murine cytomegalovirus (MCMV) is exceptionally successful in avoiding NK cell control. Here, we summarize the major MCMV evasion mechanisms targeting NK cell functions and their role in viral pathogenesis. The mechanisms by which NK cells regulate CD8+ T cell response, particularly with respect to the role of NK cell receptors recognizing viral antigens, are discussed. In addition, we discuss the role of NK cell receptors in generation and maintenance of memory NK cells. Final part of this review illustrates how the NK cell response and its viral regulation can be exploited in designing recombinant viral vectors able to induce robust and protective CD8+ T cell response.


Similar content being viewed by others
References
Biron CA, Byron KS, Sullivan JL (1989) Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 320(26):1731–1735
Loh J, Chu DT, O'Guin AK et al (2005) Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79(1):661–667
Miletic A, Krmpotic A, Jonjic S (2013) The evolutionary arms race between NK cells and viruses: who gets the short end of the stick? Eur J Immunol 43(4):867–877
Pyzik M, Gendron-Pontbriand EM, Fodil-Cornu N et al (2011) Self or nonself? That is the question: sensing of cytomegalovirus infection by innate immune receptors. Mamm Genome 22(1–2):6–18
Vidal S, Krmpotic A, Pyzik M et al (2013) Innate Immunity to Cytomegalovirus in the Murine Model. In: Reddehase MJ (ed) Cytomegaloviruses From Molecular Pathogenesis to Intervention. Caister Academic Press, Norfolk
Carayannopoulos LN, Yokoyama WM (2004) Recognition of infected cells by natural killer cells. Curr Opin Immunol 16(1):26–33
Chan CJ, Martinet L, Gilfillan S et al (2014) The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 15(5):431–438
Cannons JL, Tangye SG, Schwartzberg PL (2011) SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol 29:665–705
Horowitz A, Strauss-Albee DM, Leipold M et al (2013) Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 5(208):208ra145
Raulet DH (2003) Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 3(10):781–790
Nice TJ, Deng W, Coscoy L et al (2010) Stress-regulated targeting of the NKG2D ligand Mult1 by a membrane-associated RING-CH family E3 ligase. J Immunol 185(9):5369–5376
Lenac T, Budt M, Arapovic J et al (2006) The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J Exp Med 203(8):1843–1850
Krmpotic A, Hasan M, Loewendorf A et al (2005) NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J Exp Med 201(2):211–220
Jonjic S, Babic M, Polic B et al (2008) Immune evasion of natural killer cells by viruses. Curr Opin Immunol 20(1):30–38
Takada A, Yoshida S, Kajikawa M et al (2008) Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol 180(3):1678–1685
Diefenbach A, Jamieson AM, Liu SD et al (2000) Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 1(2):119–126
Lodoen MB, Abenes G, Umamoto S et al (2004) The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60-NKG2D interactions. J Exp Med 200(8):1075–1081
Hasan M, Krmpotic A, Ruzsics Z et al (2005) Selective down-regulation of the NKG2D ligand H60 by mouse cytomegalovirus m155 glycoprotein. J Virol 79(5):2920–2930
Loewendorf AI, Steinbrueck L, Peter C et al (2011) The mouse cytomegalovirus glycoprotein m155 inhibits CD40 expression and restricts CD4 T cell responses. J Virol 85(10):5208–5212
Lodoen M, Ogasawara K, Hamerman JA et al (2003) NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med 197(10):1245–1253
Tokuyama M, Lorin C, Delebecque F et al (2011) Expression of the RAE-1 family of stimulatory NK-cell ligands requires activation of the PI3K pathway during viral infection and transformation. PLoS Pathog 7(9):e1002265
Krmpotic A, Busch DH, Bubic I et al (2002) MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat Immunol 3(6):529–535
Ziegler H, Thale R, Lucin P et al (1997) A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6(1):57–66
Arapovic J, Lenac T, Antulov R et al (2009) Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus. J Virol 83(16):8198–8207
Zhi L, Mans J, Paskow MJ et al (2010) Direct interaction of the mouse cytomegalovirus m152/gp40 immunoevasin with RAE-1 isoforms. Biochemistry 49(11):2443–2453
Wang R, Natarajan K, Revilleza MJ et al (2012) Structural basis of mouse cytomegalovirus m152/gp40 interaction with RAE1gamma reveals a paradigm for MHC/MHC interaction in immune evasion. Proc Natl Acad Sci U S A 109(51):E3578–E3587
Moretta A, Biassoni R, Bottino C et al (2000) Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today 21(5):228–234
Kruse PH, Matta J, Ugolini S et al (2014) Natural cytotoxicity receptors and their ligands. Immunol Cell Biol 92(3):221–229
Gazit R, Gruda R, Elboim M et al (2006) Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol 7(5):517–523
Narni-Mancinelli E, Jaeger BN, Bernat C et al (2012) Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 335(6066):344–348
Stanietsky N, Mandelboim O (2010) Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett 584(24):4895–4900
Stanietsky N, Rovis TL, Glasner A et al (2013) Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol 43(8):2138–2150
Tomasec P, Wang EC, Davison AJ et al (2005) Downregulation of natural killer cellactivating ligand CD155 by human cytomegalovirus UL141. Nat Immunol 6(2):181–188
Nabekura T, Kanaya M, Shibuya A et al (2014) Costimulatory Molecule DNAM-1 Is Essential for Optimal Differentiation of Memory Natural Killer Cells during Mouse Cytomegalovirus Infection. Immunity 40(2):225–234
Engel P, Perez-Carmona N, Alba MM et al (2011) Human cytomegalovirus UL7, a homologue of the SLAM-family receptor CD229, impairs cytokine production. Immunol Cell Biol 89(7):753–766
Romo N, Magri G, Muntasell A et al (2011) Natural killer cell-mediated response to human cytomegalovirus-infected macrophages is modulated by their functional polarization. J Leukoc Biol 90(4):717–726
Zarama A, Perez-Carmona N, Farre D et al (2014) Cytomegalovirus m154 hinders CD48 cellsurface expression and promotes viral escape from host natural killer cell control. PLoS Pathog 10(3):e1004000
Chlewicki LK, Velikovsky CA, Balakrishnan V et al (2008) Molecular basis of the dual functions of 2B4 (CD244). J Immunol 180(12):8159–8167
Hengel H, Reusch U, Gutermann A et al (1999) Cytomegaloviral control of MHC class I function in the mouse. Immunol Rev 168:167–176
Kleijnen MF, Huppa JB, Lucin P et al (1997) A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J 16(4):685–694
Arase H, Mocarski ES, Campbell AE et al (2002) Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296(5571):1323–1326
Corbett AJ, Coudert JD, Forbes CA et al (2011) Functional consequences of natural sequence variation of murine cytomegalovirus m157 for Ly49 receptor specificity and NK cell activation. J Immunol 186(3):1713–1722
Farrell HE, Vally H, Lynch DM et al (1997) Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature 386(6624):510–514
Forbes CA, Scalzo AA, Degli-Esposti MA et al (2014) Ly49C-Dependent Control of MCMV Infection by NK Cells Is Cis-Regulated by MHC Class I Molecules. PLoS Pathog 10(5):e1004161
Smith HR, Heusel JW, Mehta IK et al (2002) Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A 99(13):8826–8831
French AR, **el JT, Wagner M et al (2004) Escape of mutant double-stranded DNA virus from innate immune control. Immunity 20(6):747–756
Voigt V, Forbes CA, Tonkin JN et al (2003) Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc Natl Acad Sci U S A 100(23):13483–13488
Berry R, Ng N, Saunders PM et al (2013) Targeting of a natural killer cell receptor family by a viral immunoevasin. Nat Immunol 14(7):699–705
Romasanta PN, Curto LM, Urtasun N et al (2014) A positive cooperativity binding model between Ly49 natural killer cell receptors and the viral immunoevasin m157: kinetic and thermodynamic studies. J Biol Chem 289(8):5083–5096
Carlin LE, Guseva NV, Shey MR et al (2013) The Glycophosphatidylinositol Anchor of the MCMV Evasin, m157, Facilitates Optimal Cell Surface Expression and Ly49 Receptor Recognition. PLoS One 8(6):e67295
Desrosiers MP, Kielczewska A, Loredo-Osti JC et al (2005) Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat Genet 37(6):593–599
Kielczewska A, Pyzik M, Sun T et al (2009) Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med 206(3):515–523
**e X, Stadnisky MD, Brown MG (2009) MHC class I Dk locus and Ly49G2+ NK cells confer H-2 k resistance to murine cytomegalovirus. J Immunol 182(11):7163–7171
Stadnisky MD, **e X, Coats ER et al (2011) Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood 117(19):5133–5141
Mans J, Zhi L, Revilleza MJ et al (2009) Structure and function of murine cytomegalovirus MHC-I-like molecules: how the virus turned the host defense to its advantage. Immunol Res 43(1–3):264–279
Revilleza MJ, Wang R, Mans J et al (2011) How the virus outsmarts the host: function and structure of cytomegalovirus MHC-I-like molecules in the evasion of natural killer cell surveillance. J Biomed Biotechnol 2011:724607
O'Leary JG, Goodarzi M, Drayton DL et al (2006) T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7(5):507–516
Cooper MA, Elliott JM, Keyel PA et al (2009) Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 106(6):1915–1919
Sun JC, Beilke JN, Lanier LL (2009) Adaptive immune features of natural killer cells. Nature 457(7229):557–561
Lopez-Verges S, Milush JM, Schwartz BS et al (2011) Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 108(36):14725–14732
Sun JC, Madera S, Bezman NA et al (2012) Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 209(5):947–954
Min-Oo G, Bezman NA, Madera S et al. (2014) Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J Exp Med
Foley B, Cooley S, Verneris MR et al (2012) Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 189(10):5082–5088
Min-Oo G, Kamimura Y, Hendricks DW et al (2013) Natural killer cells: walking three paths down memory lane. Trends Immunol 34(6):251–258
Hendricks DW, Balfour HH Jr, Dunmire SK et al (2014) Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol 192(10):4492–4496
Zhang T, Scott JM, Hwang I et al (2013) Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 190(4):1402–1406
Wu Z, Sinzger C, Frascaroli G et al (2013) Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol 87(13):7717–7725
Holtappels R, Pahl-Seibert MF, Thomas D et al (2000) Enrichment of immediate-early 1 (m123/pp 89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol 74(24):11495–11503
Ni J, Miller M, Stojanovic A et al (2012) Sustained effector function of IL-12/15/18- preactivated NK cells against established tumors. J Exp Med 209(13):2351–2365
Romee R, Schneider SE, Leong JW et al (2012) Cytokine activation induces human memorylike NK cells. Blood 120(24):4751–4760
Lee SH, Fragoso MF, Biron CA (2012) Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J Immunol 189(6):2712–2716
Paust S, Gill HS, Wang BZ et al (2010) Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11(12):1127–U128
Majewska-Szczepanik M, Paust S, von Andrian UH et al (2013) Natural killer cell-mediated contact sensitivity develops rapidly and depends on interferon-alpha, interferon-gamma and interleukin-12. Immunology 140(1):98–110
Peng H, Jiang X, Chen Y et al (2013) Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest 123(4):1444–1456
Dong J, Chang HD, Ivascu C et al (2013) Loss of methylation at the IFNG promoter and CNS-1 is associated with the development of functional IFN-gamma memory in human CD4(+) T lymphocytes. Eur J Immunol 43(3):793–804
Thomas M, Reuter N, Stamminger T (2013) Multiafected Regulation of Human Cytomegalovirus Gene Expression. In: Reddehase MJ (ed) Cytomegaloviruses From Molecular Pathogenesis to Intervention. Caister Academic Press, Norfolk, pp 174–195
Achour A, Baychelier F, Besson C et al (2014) Expansion of CMV-mediated NKG2C + NK cells associates with the development of specific de novo malignancies in liver-transplanted patients. J Immunol 192(1):503–511
Mitrovic M, Arapovic J, Jordan S et al (2012) The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8(+) T-cell response. J Virol 86(4):2165–2175
Andrews DM, Estcourt MJ, Andoniou CE et al (2010) Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med 207(6):1333–1343
Robbins SH, Bessou G, Cornillon A et al (2007) Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog 3(8):e123
Su HC, Nguyen KB, Salazar-Mather TP et al (2001) NK cell functions restrain T cell responses during viral infections. Eur J Immunol 31(10):3048–3055
Waggoner SN, Cornberg M, Selin LK et al (2012) Natural killer cells act as rheostats modulating antiviral T cells. Nature 481(7381):394–398
Crouse J, Bedenikovic G, Wiesel M et al (2014) Type I Interferons Protect T Cells against NK Cell Attack Mediated by the Activating Receptor NCR1. Immunity 40(6):961–973
Xu HC, Grusdat M, Pandyra AA et al (2014) Type I interferon protects antiviral CD8(+) T cells from NK cell cytotoxicity. Immunity 40(6):949–960
Andrews DM, Scalzo AA, Yokoyama WM et al (2003) Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 4(2):175–181
Lee SH, Kim KS, Fodil-Cornu N et al (2009) Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med 206(10):2235–2251
Mandaric S, Walton SM, Rulicke T et al (2012) IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence. PLoS Pathog 8(8):e1002846
Daley-Bauer LP, Roback LJ, Wynn GM et al (2014) Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe 15(3):351–362
Lang PA, Lang KS, Xu HC et al (2012) Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A 109(4):1210–1215
Hansen SG, Sacha JB, Hughes CM et al (2013) Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340(6135):1237874
Diefenbach A, Jensen ER, Jamieson AM et al (2001) Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413(6852):165–171
Slavuljica I, Busche A, Babic M et al (2010) Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest 120(12):4532–4545
Trsan T, Busche A, Abram M et al (2013) Superior induction and maintenance of protective CD8 T cells in mice infected with mouse cytomegalovirus vector expressing RAE-1gamma. Proc Natl Acad Sci U S A 110(41):16550–16555
Mohr CA, Arapovic J, Muhlbach H et al (2010) A spread-deficient cytomegalovirus for assessment of first-target cells in vaccination. J Virol 84(15):7730–7742
Cicin-Sain L, Bubic I, Schnee M et al (2007) Targeted deletion of regions rich in immuneevasive genes from the cytomegalovirus genome as a novel vaccine strategy. J Virol 81(24):13825–13834
Juranic Lisnic V, Babic Cac M, Lisnic B et al (2013) Dual analysis of the murine cytomegalovirus and host cell transcriptomes reveal new aspects of the virus-host cell interface. PLoS Pathog 9(9):e1003611
Stern-Ginossar N, Weisburd B, Michalski A et al (2012) Decoding human cytomegalovirus. Science 338(6110):1088–1093
Tsuda Y, Caposio P, Parkins CJ et al (2011) A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis 5(8):e1275
Acknowledgments
We apologize to our colleagues whose work was not cited due to space limitations. Stipan Jonjić is supported by the European Research Council (ERC) Advanced Grant (Grant number: 322693). This work has been supported in part by Croatian Science Foundation under the projects 1533 and 7132.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Immune Modulation, properties and models of CMV - Guest Editor: Ofer Mandelboim
Rights and permissions
About this article
Cite this article
Brizić, I., Lenac Roviš, T., Krmpotić, A. et al. MCMV avoidance of recognition and control by NK cells. Semin Immunopathol 36, 641–650 (2014). https://doi.org/10.1007/s00281-014-0441-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-014-0441-9