Abstract
Purpose
Cetuximab inhibits epidermal growth factor receptor (EGFR) signaling in cancer and skin cells, thereby inducing anti-cancer effects and skin disorders. The present study aimed to evaluate the relationships between serum cetuximab and EGFR-related markers, and adverse effects in head and neck cancer patients.
Methods
Thirty-four head and neck cancer patients receiving weekly intravenous cetuximab were enrolled. Serum cetuximab levels were determined just before dosing. Blood samples for determination of serum EGFR-related markers including soluble epidermal growth factor receptor (sEGFR) and interleukin-6 (IL-6) were obtained. The severities of skin disorders, their medications, and hypomagnesemia treatment were also assessed.
Results
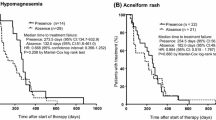
Serum levels of cetuximab and sEGFR were negatively and positively correlated with that of IL-6, respectively. The serum cetuximab level was twofold higher in the patients with a grade 2–3 skin rash than with a grade 0–1 rash. The serum cetuximab cutoff value related to severe skin rash was 71 μg/mL (sensitivity, 59%; and specificity, 94%). The use of a strong topical corticosteroid for skin rash was also associated with a higher serum cetuximab level. Serum levels of sEGFR and IL-6 had no correlations with the skin disorder severities or their medications. Hypomagnesemia treatment using intravenous magnesium sulfate was not related to serum cetuximab and EGFR-related markers.
Conclusions
Head and neck cancer patients with a higher serum IL-6 level tended to have a lower serum cetuximab level. Serum cetuximab had positive correlations to skin rash severity and its medication in the study population.




Similar content being viewed by others
Availability of data and materials
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- sEGFR:
-
Soluble EGFR
- STAT3:
-
Signal transducer and activator of transcription 3
- JAK2:
-
Janus kinase 2
- IL-6:
-
Interleukin-6
- CRP:
-
C-reactive protein
- LC–MS/MS:
-
Liquid chromatography system coupled to a tandem mass spectrometry
- ELISA:
-
Enzyme-linked immunosorbent assay
- ROC:
-
Receiver-operating characteristic
- OR:
-
Odds ratios
- 95% CI:
-
95% Confidence intervals
- IQR:
-
Interquartile range
- AUC:
-
Area under the curve
- TRPM6:
-
Transient receptor potential melastatin 6
References
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359(11):1116–1127. https://doi.org/10.1056/nejmoa0802656
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354(6):567–578. https://doi.org/10.1056/nejmoa053422
Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66(8):3992–3995. https://doi.org/10.1158/0008-5472.can-06-0191
Zimmermann M, Zouhair A, Azria D, Ozsahin M (2006) The epidermal growth factor receptor (EGFR) in head and neck cancer: its role and treatment implications. Radiat Oncol 1:11. https://doi.org/10.1186/1748-717x-1-11
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J (2017) Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 17(2):79–92. https://doi.org/10.1038/nrc.2016.126
Feng XY, Li JH, Li JZ, Han ZX, **ng RD (2010) Serum SCCA, Cyfra 21-1, EGFR and Cyclin D1 levels in patients with oral squamous cell carcinoma. Int J Biol Mark 25(2):93–98
Hudelist G, Köstler WJ, Gschwantler-Kaulich D, Czerwenka K, Kubista E, Müller R, Helmy S, Manavi M, Zielinski CC, Singer CF (2006) Serum EGFR levels and efficacy of trastuzumab-based therapy in patients with metastatic breast cancer. Eur J Cancer 42(2):186–192. https://doi.org/10.1016/j.ejca.2005.08.036
Fletcher EV, Love-Homan L, Sobhakumari A, Feddersen CR, Koch AT, Goel A, Simons AL (2013) EGFR inhibition induces proinflammatory cytokines via NOX4 in HNSCC. Mol Cancer Res 11(12):1574–1584. https://doi.org/10.1158/1541-7786.mcr-13-0187
Mojtahedi Z, Khademi B, Hashemi SB, Abtahi SM, Ghasemi MA, Fattahi MJ, Ghaderi A (2011) Serum interleukine-6 concentration, but not interleukine-18, is associated with head and neck squamous cell carcinoma progression. Pathol Oncol Res 17(1):7–10. https://doi.org/10.1007/s12253-010-9261-y
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7(4):301–311. https://doi.org/10.1016/j.ccr.2005.03.003
Bonner JA, Yang ES, Trummell HQ, Nowsheen S, Willey CD, Raisch KP (2011) Inhibition of STAT-3 results in greater cetuximab sensitivity in head and neck squamous cell carcinoma. Radiother Oncol 99(3):339–343. https://doi.org/10.1016/j.radonc.2011.05.070
Maramotti S, Paci M, Manzotti G, Rapicetta C, Gugnoni M, Galeone C, Cesario A, Lococo F (2016) Soluble epidermal growth factor receptors (sEGFRs) in cancer: biological aspects and clinical relevance. Int J Mol Sci 17(4):593. https://doi.org/10.3390/ijms17040593
Perez-Torres M, Valle BL, Maihle NJ, Negron-Vega L, Nieves-Alicea R, Cora EM (2008) Shedding of epidermal growth factor receptor is a regulated process that occurs with overexpression in malignant cells. Exp Cell Res 314(16):2907–2918. https://doi.org/10.1016/j.yexcr.2008.07.013
Zhou M, Felder S, Rubinstein M, Hurwitz DR, Ullrich A, Lax I, Schlessinger J (1993) Real-time measurements of kinetics of EGF binding to soluble EGF receptor monomers and dimers support the dimerization model for receptor activation. Biochemistry 32(32):8193–8198. https://doi.org/10.1021/bi00083a020
Baron AT, Cora EM, Lafky JM, Boardman CH, Buenafe MC, Rademaker A, Liu D, Fishman DA, Podratz KC, Maihle NJ (2003) Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomark Prev 12(2):103–113
Lemos-González Y, Rodríguez-Berrocal FJ, Cordero OJ, Gómez C, Páez de la Cadena M (2007) Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma. Br J Cancer 96(10):1569–1578. https://doi.org/10.1038/sj.bjc.6603770
Zanotti L, Paderno A, Piazza C, Pagan E, Bignotti E, Romani C, Bandiera E, Calza S, Del Bon F, Nicolai P, Ravaggi A (2017) Epidermal growth factor receptor detection in serum and saliva as a diagnostic and prognostic tool in oral cancer. Laryngoscope 127(11):E408–E414. https://doi.org/10.1002/lary.26797
Oh MJ, Choi JH, Kim IH, Lee YH, Huh JY, Park YK, Lee KW, Chough SY, Joo KS, Ku BS, Saw HS (2000) Detection of epidermal growth factor receptor in the serum of patients with cervical carcinoma. Clin Cancer Res 6(12):4760–4763
Banys-Paluchowski M, Witzel I, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer EF, Aktas B, Kasimir-Bauer S, Pantel K, Fehm T, Müller V (2017) Evaluation of serum epidermal growth factor receptor (EGFR) in correlation to circulating tumor cells in patients with metastatic breast cancer. Sci Rep 7(1):17307. https://doi.org/10.1038/s41598-017-17514-8
Wilken JA, Perez-Torres M, Nieves-Alicea R, Cora EM, Christensen TA, Baron AT, Maihle NJ (2013) Shedding of soluble epidermal growth factor receptor (sEGFR) is mediated by a metalloprotease/fibronectin/integrin axis and inhibited by cetuximab. Biochemistry 52(26):4531–4540. https://doi.org/10.1021/bi400437d
Baron AT, Wilken JA, Haggstrom DE, Goodrich ST, Maihle NJ (2009) Clinical implementation of soluble EGFR (sEGFR) as a theragnostic serum biomarker of breast, lung and ovarian cancer. IDrugs 12(5):302–308
Song JI, Grandis JR (2000) STAT signaling in head and neck cancer. Oncogene 19(21):2489–2495. https://doi.org/10.1038/sj.onc.1203483
Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS (2003) Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res 63(11):2948–2956
Squarize CH, Castilho RM, Sriuranpong V, Pinto DS Jr, Gutkind JS (2006) Molecular cross-talk between the NFκB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia 8(9):733–746. https://doi.org/10.1593/neo.06274
Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, Ackland L, Ahmed N (2009) Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer 100(1):134–144. https://doi.org/10.1038/sj.bjc.6604794
Ung N, Putoczki TL, Stylli SS, Ng I, Mariadason JM, Chan TA, Zhu HJ, Luwor RB (2014) Anti-EGFR therapeutic efficacy correlates directly with inhibition of STAT3 activity. Cancer Biol Ther 15(5):623–632. https://doi.org/10.4161/cbt.28179
Argiris A, Lee SC, Feinstein T, Thomas S, Branstetter BF 4th, Seethala R, Wang L, Gooding W, Grandis JR, Ferris RL (2011) Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral Oncol 47(10):961–966. https://doi.org/10.1016/j.oraloncology.2011.07.034
Segaert S, Van Cutsem E (2005) Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16(9):1425–1433. https://doi.org/10.1093/annonc/mdi279
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, Youssoufian H, Rowinsky EK, Ang KK (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11(1):21–28. https://doi.org/10.1016/s1470-2045(09)70311-0
Klinghammer K, Knödler M, Schmittel A, Budach V, Keilholz U, Tinhofer I (2010) Association of epidermal growth factor receptor polymorphism, skin toxicity, and outcome in patients with squamous cell carcinoma of the head and neck receiving cetuximab-docetaxel treatment. Clin Cancer Res 16(1):304–310. https://doi.org/10.1158/1078-0432.ccr-09-1928
Azzopardi N, Lecomte T, Ternant D, Boisdron-Celle M, Piller F, Morel A, Gouilleux-Gruart V, Vignault-Desvignes C, Watier H, Gamelin E, Paintaud G (2011) Cetuximab pharmacokinetics influences progression-free survival of metastatic colorectal cancer patients. Clin Cancer Res 17(19):6329–6337. https://doi.org/10.1158/1078-0432.ccr-11-1081
Liu Y, Jiang X, Gu Y, Chen Y (2019) Preventive effect of diallyl trisulfide on cutaneous toxicities induced by EGFR inhibitor. Int Immunopharmacol 69:79–87. https://doi.org/10.1016/j.intimp.2019.01.023
Flisiak I, Szterling-Jaworowska M, Baran A, Rogalska-Taranta M (2014) Effect of psoriasis activity on epidermal growth factor (EGF) and the concentration of soluble EGF receptor in serum and plaque scales. Clin Exp Dermatol 39(4):461–467. https://doi.org/10.1111/ced.12356
Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, van Cutsem E (2007) Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol 8(5):387–394. https://doi.org/10.1016/s1470-2045(07)70108-0
Shibata K, Naito T, Okamura J, Hosokawa S, Mineta H, Kawakami J (2017) Simple and rapid LC-MS/MS method for the absolute determination of cetuximab in human serum using an immobilized trypsin. J Pharm Biomed Anal 146:266–272. https://doi.org/10.1016/j.jpba.2017.08.012
Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, Eaby-Sandy B, Murphy BA (2011) Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 19(8):1079–1095. https://doi.org/10.1007/s00520-011-1197-6
Dirks NL, Nolting A, Kovar A, Meibohm B (2008) Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol 48(3):267–278. https://doi.org/10.1177/0091270007313393
Shirao K, Yoshino T, Boku N, Kato K, Hamaguchi T, Yasui H, Yamamoto N, Tanigawara Y, Nolting A, Yoshino S (2009) A phase I escalating single-dose and weekly fixed-dose study of cetuximab pharmacokinetics in Japanese patients with solid tumors. Cancer Chemother Pharmacol 64(3):557–564. https://doi.org/10.1007/s00280-008-0904-6
Yoshino T, Hasegawa Y, Takahashi S, Monden N, Homma A, Okami K, Onozawa Y, Fujii M, Taguchi T, de Blas B, Beier F, Tahara M (2013) Platinum-based chemotherapy plus cetuximab for the first-line treatment of Japanese patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: results of a phase II trial. Jpn J Clin Oncol 43(5):524–531. https://doi.org/10.1093/jjco/hyt034
Pool M, Kol A, Lub-de Hooge MN, Gerdes CA, de Jong S, de Vries EG, van Scheltinga AGT (2016) Extracellular domain shedding influences specific tumor uptake and organ distribution of the EGFR PET tracer 89Zr-imgatuzumab. Oncotarget 7(42):68111–68121. https://doi.org/10.18632/oncotarget.11827
Allen C, Duffy S, Teknos T, Islam M, Chen Z, Albert PS, Wolf G, Van Waes C (2007) Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res 13(11):3182–3190. https://doi.org/10.1158/1078-0432.ccr-06-3047
Ryman JT, Meibohm B (2017) Pharmacokinetics of monoclonal antibodies. CPT Pharmacomet Syst Pharmacol 6(9):576–588. https://doi.org/10.1002/psp4.12224
Roxburgh CS, McMillan DC (2014) Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 110(6):1409–1412. https://doi.org/10.1038/bjc.2014.90
Le Louedec F, Alix-Panabières C, Lafont T, Allal BC, Garrel R, Digue L, Guigay J, Cupissol D, Delord JP, Lallemant B, Alfonsi M, Aubry K, Mazel M, Becher F, Perriard F, Chatelut E, Thomas F (2019) Cetuximab pharmacokinetic/pharmacodynamics relationships in advanced head and neck carcinoma patients. Br J Clin Pharmacol 85(6):1357–1366. https://doi.org/10.1111/bcp.13907
Acknowledgements
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant number JP19H00398 and a Research Grant provided by the Japan Research Foundation for Clinical Pharmacology.
Funding
Kaito Shibata received financial support from the Japan Society for the Promotion of Science (JSPS) (KAKENHI, Grant Number JP19H00398). Takafumi Naito received funding from the Japan Research Foundation for Clinical Pharmacology.
Author information
Authors and Affiliations
Contributions
KS and TN conceptualized the study with input from SH and JK. KS and TN funded the study. KS and KS recruited patients and performed blood sampling with assistance from SH and HM. KS measured drug and biomarker levels. SH evaluated clinical symptoms. KS curated drug and biomarker level results and analyzed and interpreted data with assistance of TN and SH. KS and TN wrote the manuscript and all the coauthors reviewed and contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The present study was conducted in accordance with the Declaration of Helsinki, its amendments, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The study protocol was approved by the Ethics Committee of Hamamatsu University School of Medicine. All patients were fully informed of the scientific aim of this study and each patient provided written informed consent before study enrollment.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shibata, K., Naito, T., Hirakawa, S. et al. Correlations between serum cetuximab and EGFR-related markers, and skin disorders in head and neck cancer patients. Cancer Chemother Pharmacol 87, 555–565 (2021). https://doi.org/10.1007/s00280-020-04228-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04228-4