Abstract
Purpose
There is a little datum about the impact of paclitaxel dosage in patients undergoing drug-coated balloons (DCB) in endovascular therapy (EVT) for femoropopliteal lesions. In the current study, the authors sought to compare the clinical outcomes of low-dose (LD) and high-dose (HD) paclitaxel DCBs for patients undergoing EVT for femoropopliteal lesions in a real-world setting.
Materials and methods
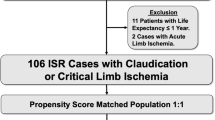
The study population was derived from a multicenter registry named “Evaluation of clinical outcome after endovascular therapy for femoropopliteal artery disease in Kanagawa” (LANDMARK registry). This registry consists of patients from 5 hospitals in Kanagawa, Japan. Overall, 1,378 patients with 1,777 lesions received treatment between July 2017 and June 2020. Among these, DCB angioplasty was performed in 477 patients (516 lesions). Propensity score matching analysis was performed to compare the clinical outcomes of LD-DCB (Lutonix; Becton Dickinson and Company, Franklin Lakes, New Jersey) and HD-DCB (IN.PACT Admiral; Medtronic Vascular, Santa Clara, CA, USA).
Results
A total of 160 matched pairs of lesions were analyzed. Primary patency and freedom from target lesion revascularization at 2 years were similar between the two groups (LD-DCB vs. HD-DCB: 72% vs. 70%, p = 0.53; and 75% vs. 73%, p = 0.59, respectively).
Conclusion
No significant differences were found in the clinical outcomes between LD-DCB and HD-DCB angioplasty for femoropopliteal lesions.
Level of Evidence
Level 3




Similar content being viewed by others
Data Availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
References
Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European stroke organization (ESO) The Task Force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J. 2018;39:763–816.
Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–88.
Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504.
Tosaka A, Soga Y, Iida O, Ishihara T, Hirano K, Suzuki K, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16–23.
Banerjee S, Sarode K, Mohammad A, Gigliotti O, Baig MS, Tsai S, et al. Femoropopliteal artery stent thrombosis: report from the excellence in peripheral artery disease registry. Circ Cardiovasc Interv. 2016;9: e002730.
Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–99.
Sheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiocasc Interv. 2014;7:10–9.
Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502.
Feldman DN, Armstrong EJ, Aronow HD, Gigliotti OS, Jaff MR, Klein AJ, et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter Cardiovasc Interv. 2018;92:124–40.
Steiner S, Schmidt A, Zeller T, Tepe G, Thieme M, Maiwald L, et al. COMPARE: prospective, randomized, non-inferiority trial of high- vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J. 2020;41:2541–52.
Boitet A, Grassin-Delyle S, Louedec L, Dupont S, Lamy E, Coggia M, et al. An experimental study of paclitaxel embolisation during drug coated balloon angioplasty. Eur J Vasc Endovasc Surg. 2019;57:578–86.
Okuno S, Iida O, Shiraki T, Fujita M, Masuda M, Okamoto S, et al. Impact of calcification on clinical outcomes after endovascular therapy for superficial femoral artery disease: assessment using the peripheral artery calcification scoring system. J Endovasc Ther. 2016;23:731–7.
Soga Y, Iida O, Hirano K, Suzuki K, Tosaka A, Yokoi H, et al. Utility of new classification based on clinical and lesional factors after self-expandable nitinol stenting in the superficial femoral artery. J Vasc Surg. 2011;54:1058–66.
Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. 2004;16:493–9.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Steiner S, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Giudice CD et al. Ranger SFA investigators. 12-month Results from the first-in-human randomized study of the ranger paclitaxel-coated balloon for femoropopliteal treatment. JACC: Cardiocasc Interv. 2018;11:934–41
Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, et al. Stella rex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcome from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102–13.
Gongora CA, Shibuya M, Wessler JD, McGregor J, Tellez A, Cheng Y, et al. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug-coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in-stent restenosis. JACC Cardiovasc Interv. 2015;8:1115–23.
Heinrich A, Engler MS, Guttler FV, Matthaus C, Popp J, Teichgraber KM. Systematic evaluation of particle loss during handling in the percutaneous transluminal angioplasty for eight different drug-coated balloons. Sci Rep. 2020;14(10):17220.
Thieme M, von Bilderling P, Paetzel C, Karnabatidis D, Delgado JP, Lichtenberg M. The 24-month results of the Lutonix global SFA Registry. JACC Cardiocasc Interv. 2017;10:1682–90.
Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, et al. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–38.
Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–53.
Micari A, Brodmann M, Keirse K, Peeters P, Tepe G, Frost M, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT global study. JACC Cardiovasc Interv. 2018;11:945–53.
Fujihara M, Higashimori A, Kato Y, Taniguchi H, Iwasaki Y, Amano T, et al. Nitinol stent implantation for femoropopliteal disease in patients on hemodialysis: results of the 3-year retrospective multicenter APOLLON study. Heart Vessel. 2016;31:1476–83.
Takahara M, Soga Y, Fujihara M, Kawasaki D, Kozuki A, Iida O. Inverse association of diabetes and dialysis with the severity of the femoropopliteal lesions and chronic total occlusion: a cross-sectional study of 2056 cases. BMC Cardiovasc Disord. 2020;20:514.
Kinstner CM, Lammer J, Willfort-Ehringer A, Matzek W, Gschwandtner M, Javor D, et al. Paclitaxel-eluting balloon versus standard balloon angioplasty in-stent restenosis of the superficial femoral and proximal popliteal artery: 1-year results of the PACUBA trial. JACC Cardiovasc Interv. 2016;9:1386–92.
Virga V, Stabile E, Biamino G, Salemme L, Cioppa A, Giugliano G, et al. Drug-eluting balloons for the treatment of the superficial femoral artery in-stent restenosis: 2-year follow-up. JACC Cardiovasc Interv. 2014;7:411–5.
Van Den Berg JC. IN-stent restenosis management: the best is yet to come. J Cardiovasc Surg. 2017;58(4):508–17.
Scheinert D, Werner M, Scheinert S, Paetzold A, Banning-Eichenseer U, Piorkowski M, et al. Treatment of complex atherosclerotic popliteal artery disease with a new self-expanding interwoven nitinol stent: 12-month results of the Leipzig SUPERA popliteal artery stent registry. JACC Cardiovasc Interv. 2013;6:65–71.
Hiramori S, Soga Y, Iida O, Suzuki K, Hirano K, Kawasaki D, et al. Relationship between clinical outcomes and vessel size in endovascular therapy for femoropopliteal lesions. J Vasc Surg. 2017;65:1690–7.
Horie K, Tanaka A, Taguri M, Inoue N. Impact of baseline and postprocedural intravascular ultrasound findings on 1-year primary patency after drug-coated balloon treatment of femoropopliteal lesions. J Endovasc Ther. 2022;29:66–75.
Lee JJ, Katz SG. The number of patent tibial vessels does not influence primary patency after nitinol stenting of the femoral and popliteal arteries. J Vasc Surg. 2012;55:994–1000.
Acknowledgements
None
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
Material preparation was performed by YI. Data collection was performed by YY, TD, KT, KH, YH, and MT. Data analysis was performed by SM and NK. The first draft of the manuscript was written by SM and MY.
Corresponding author
Ethics declarations
Conflict of Interest
Kazuki Tobita reports remuneration for lecture at Medtronic. All other authors report no conflict of interest
Ethics Approval
Approval was obtained from the local ethics committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mori, S., Yamauchi, Y., Doijiri, T. et al. Comparison Between Clinical Outcomes of Low- and High-Dose Paclitaxel Drug-Coated Balloon in Endovascular Therapy for Femoropopliteal Lesion. Cardiovasc Intervent Radiol 46, 590–597 (2023). https://doi.org/10.1007/s00270-022-03289-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03289-7